Cambridge Healthtech Institute’s Inaugural
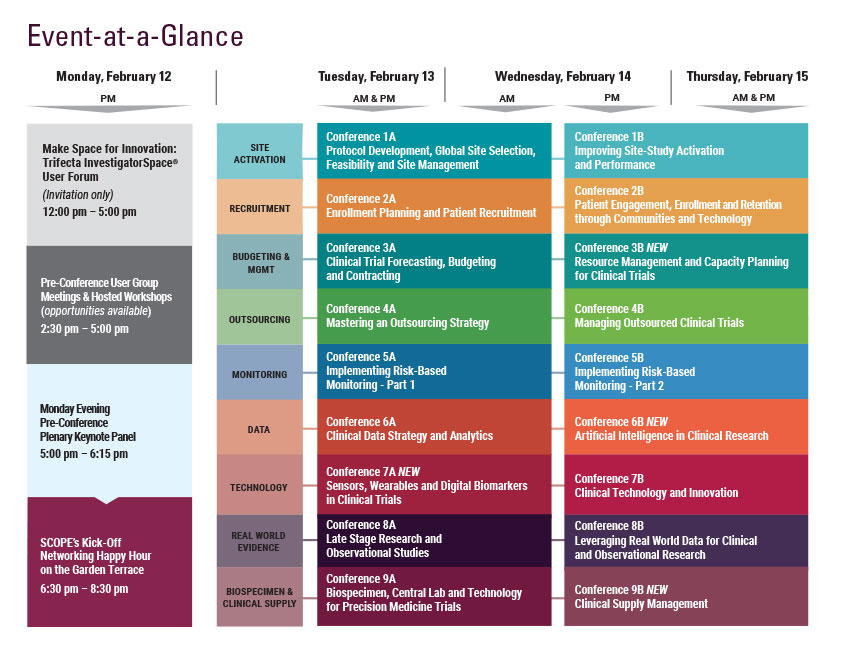
Resource Management and Capacity Planning for Clinical Trials:
Metrics and Strategies for Efficient Resource Forecasting and Management
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
Resource management and capacity planning is an essential step in setting up clinical trials. As protocols become more complex and as more partners are being used to execute them, the need to properly manage staff, workload, and outsourced partners is
more essential than ever to run efficient clinical trials and get programs executed on time and within budget with as little variance as possible. Resource managers and capacity planners must gather input from executives in finance and those at the
portfolio level, as well as from those in clinical operations and at the project level in order to understand the scope of projects in the pipeline, the effect complex protocols have on planning and timelines, and where internal and external resources
may fall short. Operations managers and the budget team must ultimately be able to find the balance between cost savings and high performance. Cambridge Healthtech Institute’s Inaugural Resource Management and Capacity Planning for Clinical Trials conference will share case studies and best practices on clinical trial finance and capacity planning, metrics for resource management algorithms, maximizing efficiency of internal-external resources, optimizing staff, and managing changes and delays.
Wednesday, February 14
11:30 am Registration Open
 12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
 Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
By leveraging site level tooling to surface nearly real-time operational metrics; sponsors and CROs are better equipped to plan for costs associated with individual sites, manage ongoing trial costs and project future budgets.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Grant Morgan, Ph.D., PMP, Senior Vice President & Head of Portfolio Planning, R&D Finance, Systems & Analytics, Portfolio & Project Management, BTG PLC
4:05 An Analytical Approach to Making Informed Decisions around Resource Management
 Geoff Kremer, Director, CMR Informatics, Strategy & Operational Effectiveness, Novo Nordisk
Geoff Kremer, Director, CMR Informatics, Strategy & Operational Effectiveness, Novo Nordisk
Innovative tools and analytics are a key tool in enabling informed decision-making around resource management. This talk will discuss various ways to optimize resource management operations with analytics and data visualization.
4:30 Quantitative and Qualitative Factors for Outsourcing versus Using Internal Resources
 Chris Chan, Executive Director, R&D Finance, Finance, FibroGen, Inc.
Chris Chan, Executive Director, R&D Finance, Finance, FibroGen, Inc.
One very important decision that biopharmaceutical companies need to make is what outsourcing strategy to pursue. Given the enormous cost and inherent complexity of the drug development process, this decision may play a key role in determining
whether a company ultimately achieves its goals. This presentation will explore both quantitative (money!) and qualitative (money isn’t everything!) factors that companies should consider when determining the right outsourcing strategy.
 4:55 Fostering a Committed Organization for Clinical Operations
4:55 Fostering a Committed Organization for Clinical Operations
Geert Vanhove, Partner, Bluecrux
Binocs is a resource planning cloud application that targets R&D, Regulatory Affairs and Laboratories. On top of the "standard" features of conventional resource planning, together with clients, we work on two innovations that drastically
speed up the organizational maturity and performance of PPM and resource planning.
5:25 Predictive Clinical Resource Planning: Overview of a Bespoke Solution (SPEAR) that Converts Planned Clinical Workload to Role-Based Resource Requirements
 Grant Morgan, Ph.D. PMP, Senior Vice President & Head of Portfolio Planning, R&D Finance, Systems &
Analytics, Portfolio & Project Management, BTG PLC
Grant Morgan, Ph.D. PMP, Senior Vice President & Head of Portfolio Planning, R&D Finance, Systems &
Analytics, Portfolio & Project Management, BTG PLC
The SPEAR Tool is used to analyze simple study information inputs to accurately predict resource requirements, visualize workload constraints, and better manage the business. This talk will cover the source data required, rationale behind
complex predictive algorithms that drive highly accurate clinical resource requirements across multiple roles and study types plus the outputs that help resource managers better plan for the future workload.
5:50 Close of Day
5:50 - 7:00 pm Track Reception (Sponsorship Opportunity Available)
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation,
we will go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
8:35 Resource Management in a Managed Network at a National Level: Lessons from the NIHR Clinical Research Network
 Divya Chadha Manek, Head of Business Development (Commercial), Clinical Research Network,
National Institute of Health Research
Divya Chadha Manek, Head of Business Development (Commercial), Clinical Research Network,
National Institute of Health Research
The NIHR Clinical Research Network is a government-funded research network that faces unique challenges related to resource management at a national level. This talk will discuss how resources are moved throughout the business and across
sites in order to properly support hundreds of clinical trials. We will also review how the finance model affects decision-making around resource planning and optimizing the efficiency of clinical trials across the UK.
9:00 CO-PRESENTATION: Workforce Resource Management: Managing Onboarding and Training for Key Functional Area Roles
Christine Senn, PhD, Chief Implementation Officer, IACT Health
 Liz Wool, RN, BSN, CCRA, CMT, Principal Consultant and Trainer, Barnett International
Liz Wool, RN, BSN, CCRA, CMT, Principal Consultant and Trainer, Barnett International
In today’s fast paced clinical research industry with heightened expectations of quality and qualified staff, effective on-boarding (beyond company orientation) requires a systematic review and analysis of key functional roles in
order to strategically align and allocate resources for the development of efficient-reproducible on-boarding practices across the enterprise. This session reviews methods and analysis practices for deployment of on-boarding and training
to include re-alignment when necessary, based upon organizational needs.
9:25 PANEL DISCUSSION: What Sponsors and CROs Need to Know about Site Capacity Planning
Moderator: Jim Kremidas, Executive Director, Association of Clinical Research Professionals
Panelists:
 Jeff Kingsley, CEO, IACT Health
Jeff Kingsley, CEO, IACT Health
 David Morin, Director, Research, Holston Medical Group
David Morin, Director, Research, Holston Medical Group
Sites have a unique set of challenges when developing a resource management plan for their clinical trials, a process that evades many sponsors and CROs. This panel discussion will address a number of ways sites do capacity planning, how
it differs from a sponsor or CRO strategy, and what sites wish these partners knew in order to close the gap in understanding. This panel will provide strategies for sponsors and CROs to improve their own budgets and resource management
based on this information.
- Discuss methods sites use to estimate staffing requirements
- Review tools to drive site efficiency
- Discuss the implications on current site payments (fee for service) vs. performance-based payment structures
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Charles O’Donnell, Director, Early Clinical Development Portfolio, AstraZeneca
10:35 Project Level Resourcing: A Journey of Resource Management
 Lisa Hegg, Ph.D., Vice President, Head Project Planning and Management, Project Management and Business Performance,
GSK
Lisa Hegg, Ph.D., Vice President, Head Project Planning and Management, Project Management and Business Performance,
GSK
The drug development process is complex and expensive, and the ability to accurately forecast project resource is critical to identifying and managing key touch points across a portfolio of projects. We will discuss how improving resource
capacity forecasting and integrating with planning across projects will enable us to effectively move resources and enable effective decision-making across the business to support a dynamic portfolio of medicines.
11:00 Driving Accountability for Resource Efficiencies in Clinical Development

Tara Dubois, MBA, Head, Clinical Trial Cost Management, Business Operations, Pfizer
Teams and business leaders often have great ideas about how to make clinical trial execution more efficient and reduce spend. As budgets get tighter, there is an increasing need to hold leaders accountable to delivering those efficiencies
and ensuring they translate to the bottom line. This presentation will focus on how to connect the dots from idea to implementation and assess the impacts on contracts, budgets and resource algorithms in a large complex organization.
11:25 Brief Session Break
11:35 Achieving Agile Resource Management in Big Pharma in Early Clinical Trials: Challenges and Successes in AstraZeneca Early Clinical Development
 Charles O’Donnell, Director, Early Clinical Development Portfolio, AstraZeneca
Charles O’Donnell, Director, Early Clinical Development Portfolio, AstraZeneca
The Early Clinical Development (ECD) group in AstraZeneca is novel and forming, with a need to be agile, pioneering and collaborative. I will describe the history, challenges and successes in managing capacity and resource in a large
pharma. Specifically, approaches to building and using resource algorithms will be described and our evolving approach to different clinical trial delivery models which utilize resource that is both internal and external. In addition,
the presentation will provide insight into some of the cultural challenges faced by a small clinical group that has hatched out of a big clinical organization.
12:00 pm Human-Centered Design in Clinical Trial Operations: Setting Your Team Up for Success
 Heather Baldwin, MPH, Principal Consultant, Frogbottom Consulting, LLC
Heather Baldwin, MPH, Principal Consultant, Frogbottom Consulting, LLC
Solutions to challenges in clinical research operations must be Business Viable, Technology Feasible, and Human Desirable to create real and lasting impact. Using Human-Centered Design in clinical trial operations engages the team
at the heart of operations to come up with a range of solutions to the challenges they face each day. Sponsors, CROs and sites that use HCD with their operations team would benefit by increasing job-ownership and satisfaction,
decreasing turn-over and training costs, decreasing start-up and enrollment periods, and decreasing team performance redundancies.
12:25 PANEL DISCUSSION: Operational Challenges in Resource Management and How to Overcome Them
Session Speakers
- How do financial, clinical, or project management criteria influence operational success?
- What are the most effective ways to address last minute changes or changes during a trial?
- What does the future of resource management look like as technology and tools develop?
12:50 Closing Remarks
1:00 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Kaitlin Kelleher
Kaitlin Kelleher
Conference Producer
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5498
E: kkelleher@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com