Cambridge Healthtech Institute’s 4th Annual
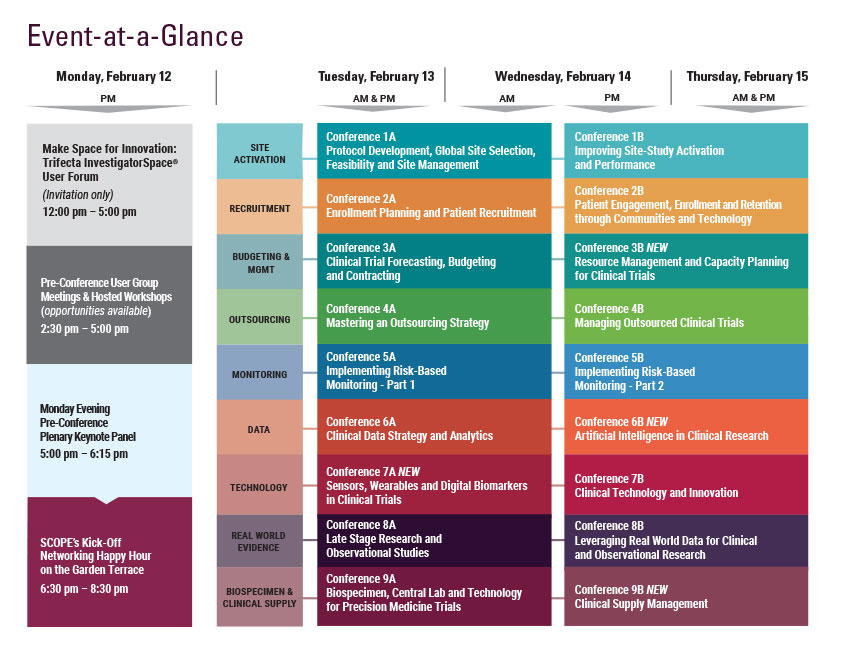
Managing Outsourced Clinical Trials
Building Successful Partnerships with Effective Oversight
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
As more clinical trial activities are outsourced to contract research organizations (CROs) and other third-party vendors, sponsors and their partners must form effective and quality partnerships. Effective management of outsourced clinical trials requires
realistic and explicit expectations from each partner in the outsourcing relationship as well as effective oversight and the ability to measure partnership and project performance and quality. Cambridge Healthtech Institute’s 4th Annual Managing Outsourced Clinical Trials conference
features case studies and lessons learned from sponsors and CROs on vendor quality and performance in light of the new ICHE6 R2 changes, as well as the outsourcing partnership and working with third party suppliers to achieve more efficient clinical
trials.
Wednesday, February 14
11:30 am Registration Open
 12:10
pm Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
12:10
pm Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
 Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
By leveraging site level tooling to surface nearly real-time operational metrics; sponsors and CROs are better equipped to plan for costs associated with individual sites, manage ongoing trial costs and project future budgets.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Walter Young, External Partner Engagement & Governance Lead, CSL Behring
4:05 Put Your Money Where Your Mouth Is: Negotiating Win-Win Contract Structures for a Successful CRO Partnership
 Sondra Smyrnios, Vice President, Clinical Operations, Alkermes
Sondra Smyrnios, Vice President, Clinical Operations, Alkermes
4:30 Talk Title to be Announced
Michael Breton, Independent Consultant
4:55 PANEL DISCUSSION: Vendor Quality and Oversight in Light of the New ICH E6 R2 Changes
 Diane Miller, Director, Vendor Management, AbbVie
Diane Miller, Director, Vendor Management, AbbVie
 Richard O’Hara, Associate Director, Clinical Outsourcing, Endo Pharmaceuticals
Richard O’Hara, Associate Director, Clinical Outsourcing, Endo Pharmaceuticals
 Cheryl Evans, Senior Vice President, Clinical & Medical Operations, CRO, Advanced Clinical
Cheryl Evans, Senior Vice President, Clinical & Medical Operations, CRO, Advanced Clinical
Rene A. Stephens, MSHS, Independent Industry Consultant
With increased pressure of the ICH E6 R2 addendum changes on quality and oversight in clinical trials, Sponsors and CROs are concerned with ensuring quality partnerships. This panel will discuss KPIs for vendor quality, quality metrics, and how Sponsors
and CROs are approaching their relationships with quality in mind.
5:50 Close of Day
5:50-7:00 pm Track Reception (Sponsorship Opportunity Available)
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation, we will
go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
Eric Forsthoffer, Vice President, Business Development, Bioclinica
8:35 Governance, Oversight and Quality Management with Key Vendors
 Sagarika Bollini, Director, Head of Clinical Partner Management, Central Clinical Planning and Solutions, Global
Clinical Operations, Bristol-Myers Squibb
Sagarika Bollini, Director, Head of Clinical Partner Management, Central Clinical Planning and Solutions, Global
Clinical Operations, Bristol-Myers Squibb
Discuss key components to CRO/sponsor relationship management and building a framework of trust within the partnership. Can we create a culture of engagement, ownership that drives performance driven culture within the partnership? This talk will focus
on governance best practices including diagnosing issues/risk and quality management frameworks for oversight.
9:00 Optimizing External Service Provider Relationships
Walter Young, External Partner Engagement & Governance Lead, CSL Behring
9:25 Supplier Segmentation and Classification: How to Make It Meaningful
Marija Nikolic, Associate Director, Vendor Management, Contracts & Outsourcing, Astellas Pharma Global Development
This presentation will focus on how to go about understanding the true business levers and drivers to make meaningful supplier segmentation. Careful consideration should be given to levers that encompass inherent business risk as well as those that drive
value. The segmentation or classification process should be made distinct to clearly drive the message as to the oversight and management of the classification. Meaningful classification translates into recognizable benefits to internal stakeholders.
9:50 Talk Title to be Announced
Rene A. Stephens, MSHS, Independent Industry Consultant
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Jens B. Thuesen, CTMS Business Development, BSI Business Systems Integration AG
10:35 Creating Stronger Biopharma-CRO Relationships Through Technology
Anca Copaescu, CEO, Strategikon Pharma
11:00 Contracting Dilemmas - Should the Sponsor or CRO Contract with Third Parties?
Lan Bandara, Director, Pharmaceutical Contract Management Group (PCMG); Director, Clinical Outsourcing, Medicines Development Centre, Coordination Department, Eisai Limited
The talk covers 1. Different contract models, 2. Pros and cons of each model with reference to new ICH E6 R2 guidelines, 3. Lessons learned from recent case studies.
11:25 Brief Session Break
11:35 pm PANEL DISCUSSION: Where Is the Industry Headed with 3rd Party Suppliers?
Bella Sessoms, Director, Development Sourcing Management, Astellas
 Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management, Medical and Development,
Astellas
Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management, Medical and Development,
Astellas
 Craig Coffman, Executive Director, Clinical Business Operations & Outsourcing, Nektar Therapeutics
Craig Coffman, Executive Director, Clinical Business Operations & Outsourcing, Nektar Therapeutics
Lan Bandara, Director, Pharmaceutical Contract Management Group (PCMG); Director, Clinical Outsourcing, Medicines Development Centre, Coordination Department, Eisai Limited
As CROs are tasked with outsourcing more services on behalf of Sponsor companies, where is the industry headed with this practice? This panel will address the following questions: Are CROs equipped to effectively outsource for additional service providers?
When Sponsors outsource to CROs, is the CRO’s capability to outsource additional services a factor in deciding to partner with a particular CRO? How is oversight and accountability of deliverables being handled? What are some best practices
and lessons learned when CROs are outsourcing multiple services?
12:50 Closing Remarks
1:00 SCOPE Summit 2018 Adjourns
For questions or suggestions about the meeting, contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com