Cambridge Healthtech Institute’s 4th Annual
Improving Site-Study Activation and Performance:
Strategically Implementing a Process for Rapid Study Start-Up
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
Clinical trial site activation and efficient study start up are critical to drug development programs, in terms of time, cost and quality of data. In order to improve start-up times and outcomes, one needs an experienced clinical research investigator, motivated and capable team members, committed partners, and efficient communication by all. Everyone (Sponsor, CRO, Site) must communicate and execute effectively in order to accelerate clinical trial timelines and optimize study site activation. As an industry, we agree that we must improve the study feasibility process, better engage our patients, standardize metrics by which we measure success, better utilize data and analytics, and develop effective patient recruitment and retention programs. In addition, embracing cross-industry initiatives to standardize and improve processes in trial start up and management and moving beyond process improvement to enable digital innovation for clinical trials (remote trials, virtual trials, mobile health) are two key trends to consider when evaluating your own approaches in your studies. Cambridge Healthtech Institute’s 4th Annual “Improving Site-Study Activation and Performance” conference will cover these issues and topics one should consider when strategically implementing a process for rapid study start-up.
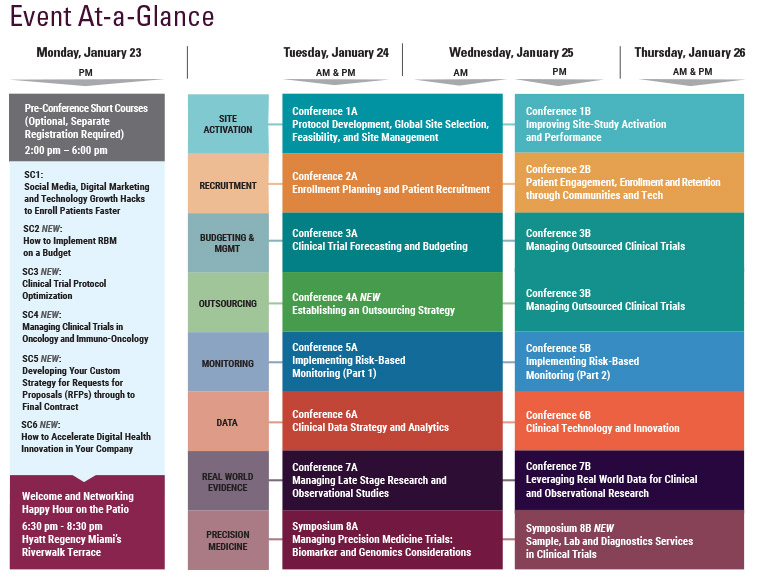
Final Agenda
Wednesday, January 25
 12:05 pm Bridging Luncheon Panel Presentation: The (Evolution) Revolution of Site Feasibility: The Current Model of Site Feasibility is Outdated, but Are We on the Brink of its New Evolution?
12:05 pm Bridging Luncheon Panel Presentation: The (Evolution) Revolution of Site Feasibility: The Current Model of Site Feasibility is Outdated, but Are We on the Brink of its New Evolution?
 Moderator: Alexandra Charge, Head, Consultative Services, Clinical Trial Optimization Solutions (CTOS), QuintilesIMS
Moderator: Alexandra Charge, Head, Consultative Services, Clinical Trial Optimization Solutions (CTOS), QuintilesIMS
Panelists: Mark Bagarazzi, CMO, Inovio Pharmaceuticals
Brendan O’Neill, Senior Director, Patient Recruitment Programs, Pfizer
Lucas Glass, Manager, Data Sciences, Analytics Centre of Excellence, QuintilesIMS
Innovation in technology and expansion of Real World Data (RWD) is substantially changing our industry, offering new approaches to the age-old operational processes in clinical development such as identification of sites and the act of surveying our investigators. With the expansion of RWD, the question now becomes could investigators’ input from the chore of site feasibility be minimized to such an extent that the only “feasibility” question remaining is whether they are interested in participating in a given study? This session aims to explore the current use of RWD in site selection and feasibility, and the future advancement in data availability and EMR technologies to further impact these processes.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Joan Chambers, COO, CenterWatch
4:05 Rethinking Relationships in Biotech with CRO Partners, Sites and Patients to Improve Outcomes
 Murray Abramson, M.D., Vice President, Global Clinical Operations, Biogen
Murray Abramson, M.D., Vice President, Global Clinical Operations, Biogen
Establishing relationships between sponsor companies and CROs can be challenging. The goals, business models, and definitions of success are different for each relationship. This presentation will focus on identifying alignment considerations that will contribute towards a more successful relationship that will meet the needs for both CRO and biotech companies.
4:30 Integrating and Leveraging Startup Documentation and Data to Speed Site Activation
 Jason Methia, Vice President, Vault Study Startup, Veeva
Jason Methia, Vice President, Vault Study Startup, Veeva
Because study startup documentation and associated data (including milestone information) are often managed in multiple systems, sponsors and CROs have little visibility into the complete startup picture. This session will explore leveraging a single system for documentation and data to actively manage and navigate through the most complex startup activities.
4:55 Participating in Industry Collaborations: Translating the External Experience into Business Practice
 Virginia Nido, Global Head, Industry Collaborations, Genentech, a member of the Roche Group
Virginia Nido, Global Head, Industry Collaborations, Genentech, a member of the Roche Group
As an industry, we are facing many challenges in drug development. There are old challenges (recruitment, site qualification, complex protocols, informed consent forms) and there are new challenges (real world data and electronic health records, mobile trials, eConsents and eLabels). How can we address these challenges and still get innovative therapies to patients efficiently? Many of us participate in industry consortia. How can we get the most out of participating in consortia by bringing the resulting solutions into our day-to-day practice? Learn how Roche and other leading pharma companies are realizing the value of industry collaborations.
5:20 INTERACTIVE PANEL: Turbocharged Site Activation for Exceptional Performance
Moderator:
 Christine Pierre, President, Society for Clinical Research Sites (SCRS)
Christine Pierre, President, Society for Clinical Research Sites (SCRS)
 Craig Lipset, MBA, Head of Clinical Innovation, R&D, Pfizer, Inc.
Craig Lipset, MBA, Head of Clinical Innovation, R&D, Pfizer, Inc.
 Dex Bilkic, MBA, Leader, Business Support Group, Boehringer Ingelheim
Dex Bilkic, MBA, Leader, Business Support Group, Boehringer Ingelheim
Site activation can be the end of an exhausting process that started well before anyone met the site and goes through to contract and regulatory packet completion. Launching the site into active performance on the study requires a new input of fuel. We’ll discuss what really works when it comes to energizing sites.

5:45 Reception hosted by Exostar
Download Brochure
Thursday, January 26
7:15 am Registration
7:30 Breakfast Co-Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap
 Christine Phillips, Senior Director, Site & Patient Access, INC Research
Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Bradford Hirsch, M.D., Co-Founder & Board Member, SignalPath, LLC
8:40 Site-Sponsor Relationships and Key Drivers of Site Performance
 Jim Kremidas, Executive Director, Association of Clinical Research Professionals (ACRP)
Jim Kremidas, Executive Director, Association of Clinical Research Professionals (ACRP)
This session will present proprietary data from a survey of sites that identifies critical aspects of successful relationships between sites and their CRO and sponsor research partners, including communication, protocols, and monitoring. This new research for the first time confirms those aspects of clinical trials that sites believe are most critical to successful site relationships and clinical trial quality, and how their CRO and sponsor partners are performing on those items.
9:05 Models to Support Different Types of PIs and Understanding the Value of Each: ACRP Certified PIs & Research Naive PIs
 David Vulcano, AVP & Responsible Executive for Clinical Research, Hospital Corporation of America (HCA)
David Vulcano, AVP & Responsible Executive for Clinical Research, Hospital Corporation of America (HCA)
This talk will present two studies, by a sponsor and by a large site organization, on determining the quality and compliance differences between certified and non-certified PIs and PIs. The first study uses protocol adherence as an endpoint and the other uses FDA audit results as an endpoint. The talk will then present an analysis useable to justify to regulators (i.e., OIG) a particular percentage increase in payments to certified PIs over those non-certified PIs. Overall, if you’re a Sponsor, CRO, SMO or site administrator interested in site quality, this is a story…with data…that you should hear.
9:30 CO-PRESENTATION: Case Study: Using Trial Participant Experience Surveys to Improve Clinical Trials
 Bert Hartog, Ph.D., Director, R&D Operations Innovation, Janssen
Bert Hartog, Ph.D., Director, R&D Operations Innovation, Janssen
 Abbe Steel, MSc, CEO, HealthiVibe, LLC
Abbe Steel, MSc, CEO, HealthiVibe, LLC
This talk will share how patient satisfaction feedback was collected via online survey upon completion of a Week 60 follow up visit of a ten-country psoriasis study. We will share first the methodology and results of a survey development study and explain how Janssen conducted a global pilot implementation study. This talk will focus on how benchmarking patient experience globally by evaluating the feasibility and value in gathering anonymous feedback from patients through a satisfaction survey has helped Janssen better understand the needs of patients and will help improve their studies in the future.
9:55 Precise and Accurate Site Selection Targeting
 Jae Chung, President & Founder, goBalto
Jae Chung, President & Founder, goBalto
Site selection is best performed using a data-driven approach to site profiling, using a weighted average of feasibility, study startup metrics, and site experience - allowing informed decisions on the effectiveness of sites, and their ability to activate, enroll patients and successfully conduct clinical trials, to be made.
10:10 Relationship Building in the Digital Age
 Brigid Flanagan, Senior Director, Clinical Development, Frenova Renal Research
Brigid Flanagan, Senior Director, Clinical Development, Frenova Renal Research
In-person investigator meetings are increasingly rare as technology depersonalizes clinical trial conduct, weakening bonds among team members. We’ll discuss why relationships are critical, ways you can set yourself apart by building personal relationships and how to integrate digital technologies and traditional communications to enhance clinical conduct and investigator performance.
10:20 Coffee Break
10:35 Chairperson’s Remarks
Jane Restorick, Chief, Business Operations, Synexus Clinical Research Ltd.
10:40 Are Your Sites Really Ready? Key Lessons for Optimizing Site Performance: Re-Engagement & Rethinking Process and Consent
 Liam Eves, Executive Vice President, Humatics, hVIVO
Liam Eves, Executive Vice President, Humatics, hVIVO
Skyrocketing development costs and increasingly complex disease indications and regulatory pathways means every minute at the investigator site counts. To meet today’s challenges, sites need to be ready to enroll the day they receive IRB approval. In order to meet this pressing business objective, sites need two critical things: an effective recruitment and workload plan, and tightly controlled oversight of that plan as it is executed. Reacting rapidly to variances against your plan can avoid months of delay further downstream in the project. This session aims to highlight effective enrolment management strategies that can help sites run more effectively, focusing in on the all-important augmentation of tactics to ensure solutions are deployed to prevent delay and keep your trial running smoothly.
10:50 Accelerating Clinical Trial Timelines by Optimizing Study Site Activation
 Navreet Dhindsa, Director, Clinical Operations, Merrimack Pharmaceuticals, Inc.
Navreet Dhindsa, Director, Clinical Operations, Merrimack Pharmaceuticals, Inc.
The amount of time spent in the start-up phase of a clinical trial can have a significant impact on overall trial timelines. Developing a strategy early-on during the planning phase of the trial can help to reduce the time spent in this phase and eventually time to data. Furthermore, site activation has a direct impact on patient enrollment and the overall study timelines, and, per some studies, it is even the direct driver of patient enrollment. This presentation will provide strategies to identify challenges early and to reduce site activation timelines.
 11:05 Improving Site Activation - From the Site’s Perspective
11:05 Improving Site Activation - From the Site’s Perspective
 Sean Stanton, Senior Vice President, Global Operations, Research Network, Bioclinica
Sean Stanton, Senior Vice President, Global Operations, Research Network, Bioclinica
With the increased start-up pressure and complex nature of studies, there tends to be a breakdown in communication. And, with the rush to use innovative, and sometimes confusing technology, the start-up process is slower and often becomes disorganized. There are better ways to speed things up and reduce the amount of complexity in a study start-up. We’ll present a site's perspective on study start-up, and share innovative approaches that address common delays in the process.
11:30 Transition to Shared Sessions
11:35 Chairperson
Matt Hendricks, Partner, Pharmica Consulting
11:40 Remote Trials: Moving beyond the Concept
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Remote Trials have been gaining more traction over the past few years as a new and innovative way to run clinical trials. The concept is certainly very interesting, but operationally very challenging to coalesce. In this talk, we will address some of these challenges, review the stakeholders perceptions around the implementation of Remote Trials, and propose the steps forward to be able to run Remote Trials in the near future.
12:05 pm CoLAB: Redefining Collaborative Engagement with Patients in Clinical Trials
 Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
The purpose of CoLAB is to improve Lilly clinical trials by considering the Site, Patient, and Patient-partner perspective. Site and Patient Simulation is one of the ways that CoLAB brings together Lilly study teams, clinical site Study Coordinators, Patients, and Patient-partners to understand real-world feedback on operational issues within our clinical protocols. By engaging your Patients upfront, you can ensure that good science aligns with good patient care. By engaging Patients early in protocol development, you can potentially improve the clinical research patient experience.
12:30 CO-PRESENTATION: Engaging with Sites and Patients to Enable Digital Innovation for Clinical Trials
 Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
 Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
In the new healthcare ecosystem and digital age, patients expect care and solutions that are coordinated, convenient, customized, and accessible. Biopharmaceutical companies are doing a lot to address these emerging expectations for patient engagement services and we are all learning a lot on the way. It is important to truly engage with sites, investigators and research volunteers using both traditional and hi-tech means and to learn from those early and ongoing interactions. With Aspire, a unique BMS effort that will be shared in this presentation, we put the focus on the Sites and Patients and the results are guiding other trial planning and management efforts.
12:55 INTERACTIVE PANEL: Digital Clinical Trial Lessons Learned: Panel Discussion from Pharma Innovators Who Have Run Virtual Trials
Moderator:
 Matt Hendricks, Partner, Pharmica Consulting
Matt Hendricks, Partner, Pharmica Consulting
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
 Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
 Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
In the past year, several large Pharma companies have begun experimenting with a new breed of reimagined clinical trials which leverage wearables and fewer sites. Now the results from the first round of these experiments are in, and the pioneers who ran the studies are ready to share their findings. Join us as we discuss what aspects of these studies are ready for prime time, where there is still work to be done, and most importantly, how patients have reacted to this shift. The conversation will focus on platforms & technology from industry veterans, startups, and established newcomers such as Apple and their ResearchKit platform.
1:20 Closing Remarks
1:25 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com