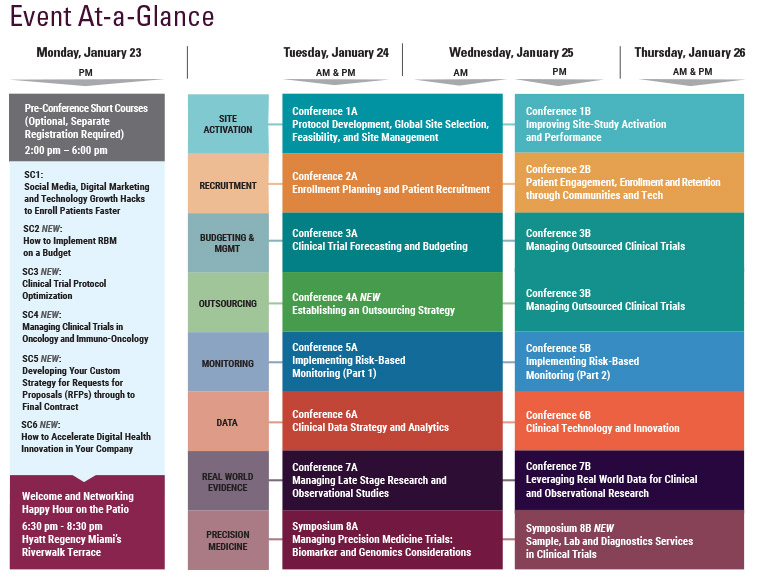
Cambridge Healthtech Institute’s 6th Annual
Managing Late Stage Research and Observational Studies:
Strategies and Technologies to Enable Non-Interventional Studies
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
Non-interventional studies are an integral part of product development plans. Product safety profiles, comparative effectiveness data as well as health economic evidence obtained from non-interventional studies, are essential for multiple stakeholders. These stakeholders include but are not limited to regulatory agencies, payers, health care management organizations, formulary inclusion decision makers, healthcare professionals, and patients. Cambridge Healthtech Institute’s 6th Annual “Managing Late Stage Research and Observational Studies" conference is designed to facilitate knowledge exchange around all aspects of observational research from the designing and managing of post-approval studies, to applying the obtained data to pivotal business and medical decisions. Similarities and differences between clinical and observational studies will be addressed by the top industry experts.
Final Agenda
1:00 pm Short Course Registration
Recommended Short Course*
2:00 – 6:00 pm Implementing Social Media, Digital Marketing and Other New Strategies for Patient Recruitment
* Separate registration required
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Rachel Edwards, Ph.D., Executive Director, Global Clinical Program Management, Amgen US
10:50 KEYNOTE: Synergy between Observational and Clinical Studies
 Cathy Critchlow, Ph.D., Vice President, Amgen Center for Observational Research
Cathy Critchlow, Ph.D., Vice President, Amgen Center for Observational Research
With the increasing integration of real world data into decision-making across the health care ecosystem, creating synergies between observational studies and both pragmatic and randomized clinical trials facilitates robust evidence generation in support of drug development and commercialization. Both pre-market and post-market observational studies have a growing role in supplementing clinical trials in regulatory submission packages. Payers are increasingly demanding replication of clinical trial results in their insured populations to enable reimbursement. However, to fully capitalize on the value and efficiencies created by an optimal portfolio of observational and clinical studies, organizational challenges due to processes associated with study design, execution and reporting that were originally created to support randomized clinical trials must be recognized and resolved. Organizational opportunities resulting from the synergies between observational and clinical studies will be discussed.
11:15 Generating Value for All Key Stakeholder: Defining Value for Patients, Assessors, Payers & Providers
 Julie C. Locklear, Pharm.D, MBA, Vice President & Head, Health Economics & Outcomes Research, EMD Serono
Julie C. Locklear, Pharm.D, MBA, Vice President & Head, Health Economics & Outcomes Research, EMD Serono
1. Changing landscape of US healthcare
2. New assessors and the value frameworks
3. Defining value across the product life cycle
11:40 Monitoring Post-Marketing Studies: Specific Features and Requirements
 Rachel Edwards, Ph.D., Executive Director, Global Clinical Program Management, Amgen US
Rachel Edwards, Ph.D., Executive Director, Global Clinical Program Management, Amgen US
Post-marketing studies are a key element of life cycle management of a product or device as they provide ongoing evidence of product benefits, risks and value in populations for whom the drug is prescribed. Studies may include clinical trials to support new indications, registries or prospective observational studies to evaluate long-term safety and chart reviews to assess drug utilization or treatment patterns. Variability in data quality and standard of evidence required drive decisions regarding the type of study monitoring needed to assure study validity, for example, fully monitored versus another model such as risk-based monitoring or real life data review. The challenges associated with trying to assure ‘fit-for-purpose’ study design and execution, including monitoring to best support study and organizational objectives, will be discussed.
12:05 pm Harnessing the Power of Real-World Data for Enhanced Real-World Study Design and Delivery
Louise Parmenter, Vice President, Epidemiology, Real World Insights, QuintilesIMS
Using real-world insights can improve study design and impact the execution and outcomes of clinical trials. Understand how to harness this data for your move healthcare forward.
12:40 Luncheon Presentation: Managed Access Programs: Design and Operational Considerations to Maximize Value
 Peggy Schrammel, Vice President, APAC and Scientific Affairs, PAREXEL ACCESS, PAREXEL
Peggy Schrammel, Vice President, APAC and Scientific Affairs, PAREXEL ACCESS, PAREXEL
Managed Access Programs (MAPs), also known as Compassionate Use, Named Patient Programs or Expanded Access, are growing in popularity, not only as a means of providing life-saving investigational therapies to needy patient populations, but also as a vital piece of the commercialization strategy. As these programs are not mandated, knowing how to design and effectively execute these programs to bring maximum value to a variety of stakeholders is key. This session will explore elements of successful design, key operational strategies that minimize cost while providing a positive investigator and patient experience, and how small additions to a core MAP strategy can be useful in supporting upcoming product commercialization goals.
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Melva Covington, Ph.D., Senior Director, Strategy Lead, Sanofi Field Medical
2:05 Synergies in the Operationalization of Clinical and Observational Studies
 Mark A. Price, MA, MEd, Senior Director, Surveys and Observational Studies, RTI Health Solutions
Mark A. Price, MA, MEd, Senior Director, Surveys and Observational Studies, RTI Health Solutions
 Lynne Hamm, Senior Director, Clinical & Medical Services, RTI Health Solutions
Lynne Hamm, Senior Director, Clinical & Medical Services, RTI Health Solutions
While randomized controlled trials are the gold standard for evaluating drug safety and efficacy, observational studies have become increasingly important in recent years to generate real world data on burden of illness, treatment patterns, health care resource utilization, safety outcomes, treatment effectiveness, adherence, and health-related quality of life among other things. These data may be used to support health technology assessments and reimbursement submissions, evaluate safety signals, or identify underserved populations or unmet needs. While clinical and observational studies share similar methodologies, there are also some notable differences. This presentation will explore these differences as well as synergies and lessons learned that could benefit scientists engaged in both clinical and observational research.
2:30 Strategic Approaches to Building Customer Engagement Models: Creating Synergizes in Real World Outcomes and Clinical Trial Evidence for Value
 Melva Covington, Ph.D., Senior Director, Strategy Lead, Sanofi Field Medical
Melva Covington, Ph.D., Senior Director, Strategy Lead, Sanofi Field Medical
This presentation will cover several key topics such as approaches to strategic planning and environment assessment to build customer engagement models that address scientific needs of payors, health care providers and patients, understanding how to navigate the risks and uncertainties to maximize engagement outcomes, leverage scientific information for alignment in a matrix environment and with external stakeholders, and build effective performance standards to evaluate impact and measure success of models.
 2:55 Managing Late Stage Research, Observation Studies & Registries
2:55 Managing Late Stage Research, Observation Studies & Registries
 Christina Fawcett, PMP, Director of US Operations, Late Phase Services, PRA Health Sciences
Christina Fawcett, PMP, Director of US Operations, Late Phase Services, PRA Health Sciences
The heterogeneity of late-phase study designs, combined with an increased use of alternative monitoring strategies and an evolving regulatory framework collectively warrants careful operational planning. Early consultation and alignment of stakeholder groups to define protocol-specific study goals and data use, the regulatory strategy to be employed, and clinical rigor to be implemented under ICH/GxP guidances are key considerations. Increasingly, innovative patient-facing technologies are being integrated into global late-phase study designs to support streamlined data capture, reduce stakeholder burden, and increase value creation. The intersection of these operational parameters will be discussed.
3:20 PANEL DISCUSSION: Meeting the Evidentiary Needs of Multiple Stakeholders by Better Non-Interventional Studies
Moderator:  Cathy Critchlow, Ph.D., Vice President, Amgen Center for Observational Research
Cathy Critchlow, Ph.D., Vice President, Amgen Center for Observational Research
Panelists:
Rachel Edwards, Ph.D., Executive Director, Global Clinical Program Management, Amgen US
Julie C. Locklear, Pharm.D., MBA, Vice President & Head, Health Economics & Outcomes Research, EMD Serono
Christina Fawcett, PMP, Director of US Operations, Late Phase Services, PRA Health Sciences
Topics to be discussed include but are not limited to the following:
- What are key considerations and approaches to balance scientific and commercial values of non-interventional studies?
- What are common utilization of non-interventional studies in supporting clinical development programs?
- How can evidences generated from non-interventional studies be used in discussions with regulatory agencies during product development and post-marketing in support of establishing product benefit risk profile, continual safety monitoring, and risk management and mitigation activities, as well as fulfilling regulatory post-marketing safety requirement (PMRs and FUMs)?
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Download Brochure
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
William Spalding, Ph.D., Director, Epidemiology Lead in Global Health Economics and Outcomes Research and Epidemiology, Shire
8:30 Building Differentiated Value Propositions in an Evolving Environment
 Riad Dirani, Ph.D., Vice President, Global Health Economics and Outcomes Research (GHEOR), Teva Pharmaceuticals
Riad Dirani, Ph.D., Vice President, Global Health Economics and Outcomes Research (GHEOR), Teva Pharmaceuticals
With the continued shift towards a value-focused healthcare system, it has become even more critical for pharma companies to build solid value propositions for their products and offerings. An important component of this approach is developing a sound, scientifically robust research program utilizing real-world evidence (RWE) as well as P4 planning in the peri-launch phase. This presentation will provide an overview of this approach and examples of its application.
8:55 Opportunities and Challenges in the Use of RWE to Support Product Value Propositions
William Spalding, Ph.D., Director, Epidemiology Lead in Global Health Economics and Outcomes Research and Epidemiology, Shire
Data sources for RWE studies have evolved from use of administrative claims databases to assess disease burden and treatment outcomes, to use of anonymized electronic health records that include contextualized physician notes. While EHR provides a robust data source that goes beyond that available via administrative claims, they have their own unique methodological challenges. This talk will focus on some of these challenges, and explore potential next-step evolution in RWE originating out of EHR studies.
9:20 Patient Access and Outreach: The Role of Disease Foundations to Secure and Manage Real World Data
 Ginger Spitz, Executive Director, Foundation of Sarcoidosis Research
Ginger Spitz, Executive Director, Foundation of Sarcoidosis Research
This presentation will focus on the valuable role of non-profit disease research foundations in securing and managing real world data through patient registries, clinical trial network, and recruitment. Given the “neutral third party” status of theses non-profit organizations, issues in compliance and logistics can be navigated more easily. In addition, unlike CROs, foundations are more likely to provide viable and applicable patient information and outreach. This presentation will discuss registries, patient member databases, networks, social media outreach, and other techniques for securing and managing data.
9:45 Optimizing Operations in Post-Approval Research Requires a New Way of Thinking
 Kirsten Colling, Senior Director Global Operations, Post-Approval Research, Bioclinica
Kirsten Colling, Senior Director Global Operations, Post-Approval Research, Bioclinica
Post-approval research studies are becoming increasingly large and complex in the post-approval clinical research landscape. With the pressing need to obtain real-world data, assess product safety profiles, and support the full span of a product’s lifecycle, the ability to conduct efficient, cost-effective post approval studies is more important than ever. It’s time to bring post-approval operational strategies into the 21st century.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Sean Zhao, M.D., Head, US Patient Safety Surveillance, AstraZeneca
11:15 Strategic and Practical Considerations in Combining Clinical Trials and Observational Studies for Product Safety Profile Assessment
Sean Zhao, M.D., Head, US Patient Safety Surveillance, AstraZeneca
Proactively assess medicinal product’s safety profile is a critical success factor in optimizing benefit and minimizing risk of patients receiving treatment of a pharmaceutical product in clinical practice. However, pharmaceutical companies commonly face great challenges in assessing such safety profile effectively during clinical trials and early phase of marketing shortly after a product launch because of limitations of clinical trials and lack of clinical practice data in drug utilization and treatment effects. In addition, pharmaceutical companies have to fulfil post marketing regulatory risk assessment requirements for newly approved medicinal products. By combining clinical trials and observational studies, pharmaceutical companies may be able to overcome above limitations and challenges, to effectively and efficiently assess product safety profile and to fulfil regulatory’ s post marketing safety requirements in the early phase of product marketing. This presentation will discuss strategic thinking and practical considerations in how to combine clinical trials and observational studies to assess product risk profile during early phase of product marketing.
11:40 Leveraging Real-World Observational Data for Safety Contextualization throughout a Product’s Life Cycle: A Case Study
 Jamie Geier, Ph.D., Senior Director Epidemiology, Epidemiology, Pfizer
Jamie Geier, Ph.D., Senior Director Epidemiology, Epidemiology, Pfizer
While many randomized clinical trials include at least one control group, the size of the control groups and duration of treatment may not permit precise comparative assessments for adverse events with low frequency or long latency. The use of indirect comparative methods can provide such context, but must take into account potential differences in the populations compared whose characteristics may vary. Data from observational sources can be used to provide these comparisons. This discussion will highlight approaches to address the strengths and weaknesses of observational data sources via a case study.
12:05 pm Bridging Luncheon Presentation: Leveraging Educational Materials in the Site and Patient Engagement for Observational Research
 Heather Gartman, Regional Managing Director, Public Relations Group, inVentiv Health
Heather Gartman, Regional Managing Director, Public Relations Group, inVentiv Health
 Julie Randolph, Ph.D., Project Director, Phase IV Operations, inVentiv Health
Julie Randolph, Ph.D., Project Director, Phase IV Operations, inVentiv Health
Researchers must be highly attuned to an increasingly engaged, well-informed, and metric savvy patient population. Developing meaningful research relationships with patients drives successful real-world evidence generation.
-Spark patient interest in observational research opportunities by demonstrating participation value
-Explore physician-patient connectivity strategies driving ongoing engagement
-Strengthen patient-site relationships via multiple points-of-contact
-Analyze patient feedback from recent clinical research experience
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com