Cambridge Healthtech Institute’s Inaugural
Sample, Lab and Diagnostics Services in Clinical Trials:
Infrastructure to Support Biomarker-Driven Trials
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
The availability of high quality biological specimens, laboratory access and diagnostics services are of utmost importance for biomarker-driven clinical trials and future research. The complexity and number of samples collected during studies has increased steadily over the years and we need to come up with best practices, operational models and IT systems to deal with this volume and complexity. The next step, the testing of the samples and various laboratory services also require significant managerial efforts whether they are outsourced or provided by an in-house laboratory. The 2017 "Sample, Lab and Diagnostics Services in Clinical Trials" symposium brings together leading experts, representing clinical sponsors, to discuss challenges and identify actions to improve infrastructure for biomarker driven clinical trials.
Scientific Advisor: Brenda Yanak, Ph.D., Director, Precision Medicine Leader, Clinical Innovation, Pfizer
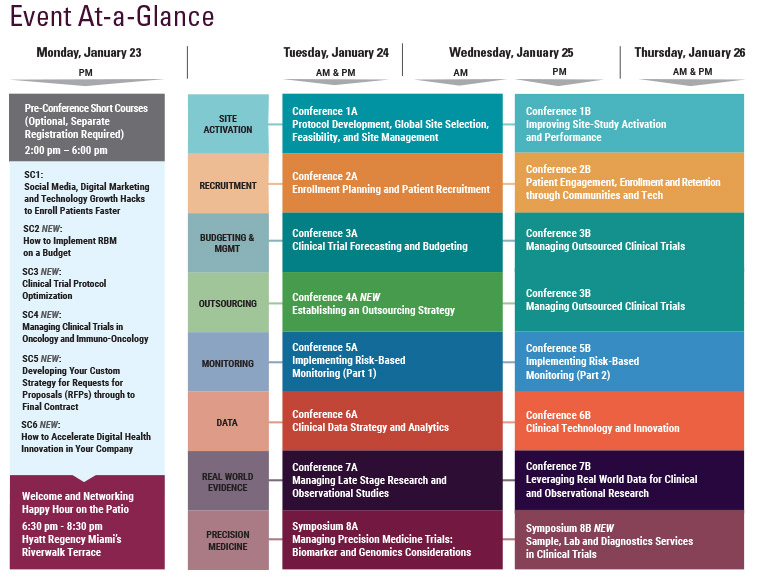
Final Agenda
Arrive early and attend Part 1: Managing Precision Medicine Trials.
Wednesday, January 25
12:10 pm Lunch on Your Own
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Rebecca Simamora, Senior Director, Clinical Operations Head, MedImmune
4:05 Co-Presentation:Leverage Strategic CRO Partnership and Technology Innovation to Modernize Clinical Sample Management and Compliance in Managing Precision Medicine Trials
 Rebecca Simamora, Senior Director, Clinical Operations Head, MedImmune
Rebecca Simamora, Senior Director, Clinical Operations Head, MedImmune
 Daniel Joelsson, Director, Global Business Planning & Operations, MedImmune
Daniel Joelsson, Director, Global Business Planning & Operations, MedImmune
With the shift towards biomarker-driven trials and precision medicine, the complexity and number of samples collected during studies has increased steadily. At the same time, the operational models and IT systems to deal with this complexity haven’t always kept pace. More and more internal resources are being spent on sample logistics, resources that could be better spent managing studies. This talk will show that by creating strategic partnerships with central labs and implementing an innovative technology solution, those resources can be freed up to focus on driving pipeline projects forward.
5:05 Interactive Discussion: Biospecimen Handling Technology and IT Framework in Biomarker-Driven Trials
Rebecca Simamora, Senior Director, Clinical Operations Head, MedImmune
Daniel Joelsson, Director, Global Business Planning & Operations, MedImmune
Topics to be discussed include, but are not limited to, the following:
- Clinical informatics solutions for additional logistics challenges of biomarker-driven clinical trials
- Managing sample collection to support clinical trials
- Assuring the quality and the sample-test-result flow
- Addressing the issues related to global trials
- Widely available and in-house IT application for sample management
5:45 Close of Day
Download Brochure
Thursday, January 26
7:15 am Registration
7:45 Morning Coffee
8:35 Chairperson’s Remarks
Audrey Plough, Executive Director, Operations, Clinical Research Operations, Immune Tolerance Network, University of California, San Francisco
8:40 GSK Biological Sample Management Strategy
 Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology & Science, GSK
Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology & Science, GSK
GSK is executing a strategy to maximize investment in biomaterials including clinical trial subject samples. As such, we are rationalizing our storage strategy for short, medium and long term storage by creating life cycle management and centralizing on-site materials where shared and secondary use creates scientific value.
- Standardizing storage formats and technology
- Automated storage systems to reduce effort for delivering just-in-time samples
- Legacy IT landscape challenges
- Considerations for shared and secondary use
9:10 Management of Biospecimens Collected in Complex Biomarker-Driven Clinical Trials: Convergence of IT Solutions with Classical Clinical Practice
 Michael Tanen, Director, Clinical BMx Specimen Management, Merck Research Laboratories
Michael Tanen, Director, Clinical BMx Specimen Management, Merck Research Laboratories
Recent progress in translational medicine has created the need for new and innovative ways to manage clinical biospecimens. The demand to develop systems and processes to manage the requirements of integration, consent, data sharing, and association with clinical data has pushed the traditional approaches of past generation biorepository systems into the 21st century. The next-generation biorepository will need to be integrated, agile, and provide an intuitive user experience that can drive greater access to, better decisions from, and foster comprehensive information that will lead to improvements in patient care. The ability to access and generate the right data at the right time will become standard as we move into a period of precision medicine and individualized care.
9:40 Sample Management: 10 Essential Elements of a Sample Management Plan
 Audrey Plough, Executive Director, Operations, Clinical Research Operations, Immune Tolerance Network, University of California, San Francisco
Audrey Plough, Executive Director, Operations, Clinical Research Operations, Immune Tolerance Network, University of California, San Francisco
This presentation will focus on sharing the Immune Tolerance Network’s (ITN) sample management experience and lessons learned over the past 17 years by providing an overview of the ITN’s sample collection, tracking, and QA/QC processes and systems which have been applied to ensure specimen quality, data standardization by minimizing inter-operator/inter-site variability, and sample data discrepancies.
10:10 Coffee Break
10:35 Chairperson’s Remarks
Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol Meyers-Squibb
10:40 Central Lab Sourcing: Challenges and Solutions
 Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol Meyers-Squibb
Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol Meyers-Squibb
Methodology for selection of Central Lab partners in a changing marketplace. Navigating the diverse needs of Central Lab stakeholders and translating needs into business requirements and selection criteria. Flexibility in sourcing strategy to account for growth in technology and capabilities
11:15 Best Practices: Companion Diagnostic and Biomarker Lab Management
 Shruthi Sampath, Biomarker Operations Program Leader, Genentech
Shruthi Sampath, Biomarker Operations Program Leader, Genentech
- General roles and responsibilities
- Working with a diagnostic partner and shared lab oversight
- Global Study set-up and ethics/compliance considerations
- Operational considerations
- Regulatory landscape
- Case study
11:50 Outsourcing Tissue Histopathology Investigations in Support of Clinical Trials for Novel Therapeutics: Considerations and Perspectives
 Keith Wharton, Ph.D., Molecular Pathologist, Preclinical Safety, Novartis
Keith Wharton, Ph.D., Molecular Pathologist, Preclinical Safety, Novartis
Tissue histopathology investigations are central to discovery and preclinical development of novel therapeutics and routine medical care, but their variable use in human clinical trials represents a missed opportunity to improve our understanding of disease and the effects of various therapies on disease. Here we discuss, within a question-based framework, major considerations when implementing tissue histopathology biomarker investigations in clinical trials for novel therapeutics.
12:25 pm Interactive Discussion: Biobanks to Serve Clinical Trials
Moderator: Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol Meyers-Squibb
Panelists:
Michael Tanen, Director, Clinical BMx Specimen Management, Merck Research Laboratories
 Kamala K Maddali DVM, Ph.D., VP, Biopharm Market Development, Collaborations and Companion Diagnostics, Cancer Genetics, Inc.
Kamala K Maddali DVM, Ph.D., VP, Biopharm Market Development, Collaborations and Companion Diagnostics, Cancer Genetics, Inc.
 Rob Fannon, MBA, MPH, Vice President of Operations,Cancer Genetics, Inc.
Rob Fannon, MBA, MPH, Vice President of Operations,Cancer Genetics, Inc.
Topics to be discussed include, but are not limited to, the following:
- Operational considerations: Sample procurement, identification and de-identification
- Regulatory considerations
- Running in-house biobanks
- Partnering with commercial biobanks
12:50 Closing Remarks
12:55 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
Download Brochure
Arrive early and attend Part 1: Managing Precision Medicine Trials
Keynotes | Monday Short Courses | Speaker Biographies
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com