Cambridge Healthtech Institute’s 6th Annual
Clinical Technology and Innovation:
Digital Technologies and Novel eClinical Solutions
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
Digital technology, mobile solutions, novel data collection modalities and integrative systems are becoming game-changing features of modern clinical trials. However, the adoption of novel technology solutions to improve overall outcomes and garner operational efficiencies, has been slower than expected. Cambridge Healthtech Institute’s 6th Annual “Clinical Technology and Innovation” conference will feature a broad array of topics such as digital biomarkers, wearables, cloud solutions, novel data visualization, and their adoption and implementation in clinical research. We are looking forward to hosting a practical and productive knowledge and experience exchange.
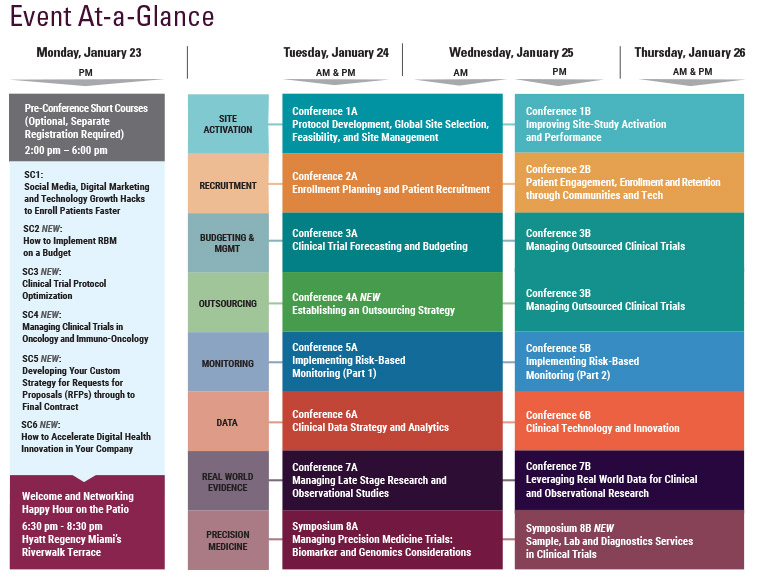
Final Agenda
Arrive early and attend Part 1: Clinical Data Strategy and Analytics.
Wednesday, January 25
 12:05 pm Bridging Luncheon Co-Presentation: Bridging the Clinical Data Structure Gap for Holistic RBM: How Fully Integrated Data Empowers Risk Management
12:05 pm Bridging Luncheon Co-Presentation: Bridging the Clinical Data Structure Gap for Holistic RBM: How Fully Integrated Data Empowers Risk Management
 Sudeep Pattnaik, MS, MBA, Co-Founder & CEO, ThoughtSphere, Inc.
Sudeep Pattnaik, MS, MBA, Co-Founder & CEO, ThoughtSphere, Inc.
 Pankaj Manon, Co-Founder & CTO, ThoughtSphere, Inc.
Pankaj Manon, Co-Founder & CTO, ThoughtSphere, Inc.
Learn how a purpose-built for clinical trials data lake and informatics solution lets you take advantage of leading-edge big data practices for holistic RBM, removing the data structure barrier. Data integration brings to life risks that are not apparent when you examine the data from one source alone. Now accessing all clinical data regardless of its format in near real-time is possible, available in one place enabling actionable insights.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Jaydev Thakkar, Clinical Trial Design and Management, Global IS Service Owner, Director Information Systems, Amgen
4:05 Co-Presentation: Transforming Clinical Trials and Patient Engagement with Digital Health Innovations – Wearables, Sensors and Mobile Apps
 Jaydev Thakkar, Clinical Trial Design and Management, Global IS Service Owner, Director Information Systems, Amgen
Jaydev Thakkar, Clinical Trial Design and Management, Global IS Service Owner, Director Information Systems, Amgen
Sindhya Govind, IS Lead, Clinical Trial Patient Engagement Technologies, Amgen
Is all the buzz around Digital Health a hype or is there a real opportunity to transform clinical trials and leverage technology for increased patient engagement and retention? This presentation will take you on a journey to explore use of Digital Health innovations in clinical trials and share lessons learned from initial pilots.
4:30 Clarity through Connectivity
 Andrew Masters, Senior Vice President, Chief Technology Officer, eHealth Solutions, Bioclinica
Andrew Masters, Senior Vice President, Chief Technology Officer, eHealth Solutions, Bioclinica
There is a lot of talk about trial data, and much of it about quantity and variety. But what can we learn? Successful clinical trials require the ability to see key details and uncover hidden insights, and the best way to accomplish this is through connectivity. This session, delivered by the man responsible for one of the most dynamic technology platforms in the industry, will show the value of connectivity and clarity in clinical trials.
4:55 The Current eSource Landscape: TransCelerate eSource Initiative Activities
Ed Kellar, Director, Global Data Management Operational Support, Astellas Pharma Global Development, Inc
The TransCelerate eSource Initiative intends to work towards the optimization of electronic data sources to improve global clinical science and global clinical trial execution for patients, sites, and sponsors. The first step in that process is to evaluate the eSource landscape. TransCelerate will share results of two surveys and other stakeholder engagements that provide insight into what is already happening and what needs to be done to accelerate the move to an electronic source environment globally. Demonstration projects, which will be developed from specific use cases, will also be shared.
5:20 Co-Presentation: Robotics & Process Automation in Clinical Development: An AbbVie Case Study
 Aman Thukral, Assistant Director, DSS, AbbVie
Aman Thukral, Assistant Director, DSS, AbbVie
Nareen Katta,Director, Operations Analytics, Data Sciences, Data and Statistical Sciences, AbbVie
Embarking on the theme of efficiency and productivity, AbbVie piloted robotics in one of the clinical development function. Currently, the data sciences group creates accounts for investigators and site staff in the Interactive Response Technology (IRT) system by entering information manually using the admin module. Before the creation of the account in the IRT system, additional due diligence is performed if site staff has completed the required GxP training for access. The process is labour-intensive, time-consuming, and error-prone. To mitigate these challenges, robotics software was deployed that used human credentials and worked on the front end admin module to create accounts. The robot also ensured the training and other requirements before access was provided. This yielded several benefits:The proposed case study is based on novel technology trend, which is emerging in other industries, but yet to make a stride in pharmaceutical R&D
5:45 Close of Day
Thursday, January 26
7:15 am Registration
7:30 Co-Breakfast Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap

Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Kuno van der Post, Senior Vice President, Business Development, OmniComm Systems, Inc.
8:40 mClinical Is Changing the Way We Execute Clinical Trials
 Munther Baara, Senior Director, Development Business Technology, Pfizer
Munther Baara, Senior Director, Development Business Technology, Pfizer
There is no day that passes by where we don’t hear about new technology and initiatives helping business bridge the gap and evolve the organization though digital transformation, I will share with you:
- An enterprise vision for mobile tools supporting the development portfolio
- Delivering the user experience that study participants deserve
- Making the case with internal stakeholders
- Functionality for today and tomorrow
9:05 Trends and Utility of Virtual Technology in Clinical Trials
 Yechiel Engelhard, M.D., MBA, Senior Director Patient Technologies, Teva
Yechiel Engelhard, M.D., MBA, Senior Director Patient Technologies, Teva
Traditional site-centric trials are designed around the benefits and limitations of the site itself, and aggregate data in a sporadic and retrospective nature. Virtual trials are designed with and for the patient, with wearable and digital devices helping to provide remote, continuous monitoring throughout the trial. This leads to more objective and efficient data collection. This talk aims to explore the user perception and system benefits of wearable and digital devices in a clinical trial setting.
9:30 Complementing Clinical Trials with Digital Biomarkers from Real World Smartphone Data: Science or Still Fiction
 Christian Gossens, Ph.D., Global Head Early Development Workflows, pRED Informatics, Roche Pharmaceutical Research and Early Development
Christian Gossens, Ph.D., Global Head Early Development Workflows, pRED Informatics, Roche Pharmaceutical Research and Early Development
Objective and high frequency measures of disease progression strengthen the clarity of clinical endpoints. Two years ago, we started into digital biomarker research for Parkinson’s Disease by providing patients in an interventional clinical trial with mobile sensors they carry day in and day out. This presentation will discuss how this data from outside the clinic is complementing the standard clinical data captured during normal site visits.
 9:55 Going Beyond Integration – Centralizing Data with Purpose
9:55 Going Beyond Integration – Centralizing Data with Purpose
 Nikhil Gopinath, Senior Solutions Engineer, Life Sciences Business Consulting, Saama Technologies, Inc.
Nikhil Gopinath, Senior Solutions Engineer, Life Sciences Business Consulting, Saama Technologies, Inc.
Clinical data repositories have evolved given the recent push to integrate numerous clinical data assets from trials. With the advent of new digital information, (e.g. wearables, OMICS, & real world) there is a data-deluge that presents new challenges to clinops stakeholders. Saama presents a point-of-view on leveraging integrative technologies to drive outcomes in multiple facets of the clinical trial landscape. Areas of focus include: leveraging real world data for feasibility analysis and enhanced study monitoring.
10:20 Coffee Break
10:35 Chairperson’s Remarks
Munther Baara, Senior Director, Development Business Technology, Pfizer
10:40 Closing Our Clinical Trial Databases within 72 Hours of LPLV
 Nina Spiller, PharmD, Vice President, Clinical Management, Otsuka Pharmaceutical Development & Commercialization, Inc.
Nina Spiller, PharmD, Vice President, Clinical Management, Otsuka Pharmaceutical Development & Commercialization, Inc.
Otsuka has used eSource technology in nine trials over the last several years. We have seen a number of significant differences in the conduct of these trials which has led to a corporate-wide objective of accelerating our timelines by closing our study databases within 3 days of the last patient visit.
 11:05 The Future of IRT is in Your Hands — Leveraging Mobile Technology to Bring IRT into the 21st Century
11:05 The Future of IRT is in Your Hands — Leveraging Mobile Technology to Bring IRT into the 21st Century
 Kelly Knowles, PMP, Director, Client Services, Bracket
Kelly Knowles, PMP, Director, Client Services, Bracket
The future of IRT is in your hands - leveraging Mobile Technology to bring the full value of IRT from inventory management to patient engagement. Bracket will present The Future of IRT is in Your Hands – Leveraging Mobile Technology to Bring Clinical IRT into the 21st Century. Some discussion topics include the mobile landscape, mobile apps for clinical trials, delivering quick study metrics, and patent engagement.
11:30 Transition to Shared Sessions
11:30 Chairperson's Remarks
Matt Hendricks, Partner, Pharmica Consulting
11:35 Remote Trials: Moving beyond the Concept
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Remote Trials have been gaining more traction over the past few years as a new and innovative way to run clinical trials. The concept is certainly very interesting, but operationally very challenging to coalesce. In this talk, we will address some of these challenges, review the stakeholders perceptions around the implementation of Remote Trials, and propose the steps forward to be able to run Remote Trials in the near future.
12:05 pm CoLAB: Redefining Collaborative Engagement with Patients in Clinical Trials
 Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
The purpose of CoLAB is to improve Lilly clinical trials by considering the site, patient, and patient-partner perspective. Site and patient simulation is one of the ways that CoLAB brings together Lilly study teams, clinical site study coordinators, patients, and patient-partners to understand real-world feedback on operational issues within our clinical protocols. By engaging your patients upfront, you can ensure that good science aligns with good patient care. By engaging patients early in protocol development, you can potentially improve the clinical research patient experience.
12:30 CO-PRESENTATION: Engaging with Sites and Patients to Enable Digital Innovation for Clinical Trials
 Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
 Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
In the new healthcare ecosystem and digital age, patients expect care and solutions that are coordinated, convenient, customized, and accessible. Biopharmaceutical companies are doing a lot to address these emerging expectations for patient engagement services and we are all learning a lot on the way. It is important to truly engage with sites, investigators and research volunteers using both traditional and hi-tech means and to learn from those early and ongoing interactions. With Aspire, a unique BMS effort that will be shared in this presentation, we put the focus on the Sites and Patients and the results are guiding other trial planning and management efforts.
12:55 INTERACTIVE PANEL: Digital Clinical Trial Lessons Learned: Panel Discussion from Pharma Innovators Who Have Run Virtual Trials
Moderator:
 Matt Hendricks, Partner, Pharmica Consulting
Matt Hendricks, Partner, Pharmica Consulting
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
 Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
 Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
In the past year, several large Pharma companies have begun experimenting with a new breed of reimagined clinical trials which leverage wearables and fewer sites. Now the results from the first round of these experiments are in, and the pioneers who ran the studies are ready to share their findings. Join us as we discuss what aspects of these studies are ready for prime time, where there is still work to be done, and most importantly, how patients have reacted to this shift. The conversation will focus on platforms & technology from industry veterans, startups, and established newcomers such as Apple and their ResearchKit platform.
1:20 Closing Remarks
1:25 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com