Cambridge Healthtech Institute’s 7th Annual
Late Stage Research and Observational Studies:
Novel Approaches and Data Sources for Post-Approval Research
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Non-interventional studies are an integral part of product development plans. Product safety profiles, comparative effectiveness data, as well as health economic evidence obtained from non-interventional studies, are essential for multiple stakeholders.
These stakeholders include but are not limited to regulatory agencies, payers, health care management organizations, formulary inclusion decision makers, healthcare professionals, and patients. Cambridge Healthtech Institute’s 7th Annual Late Stage Research and Observational Studies:
Novel Approaches and Data Sources for Post-Approval Research conference is designed to facilitate knowledge exchange around all aspects of observational research from the designing and managing of post-approval studies, to applying
the obtained data to pivotal business and medical decisions. Similarities and differences between clinical and observational studies will be addressed by the top industry experts.
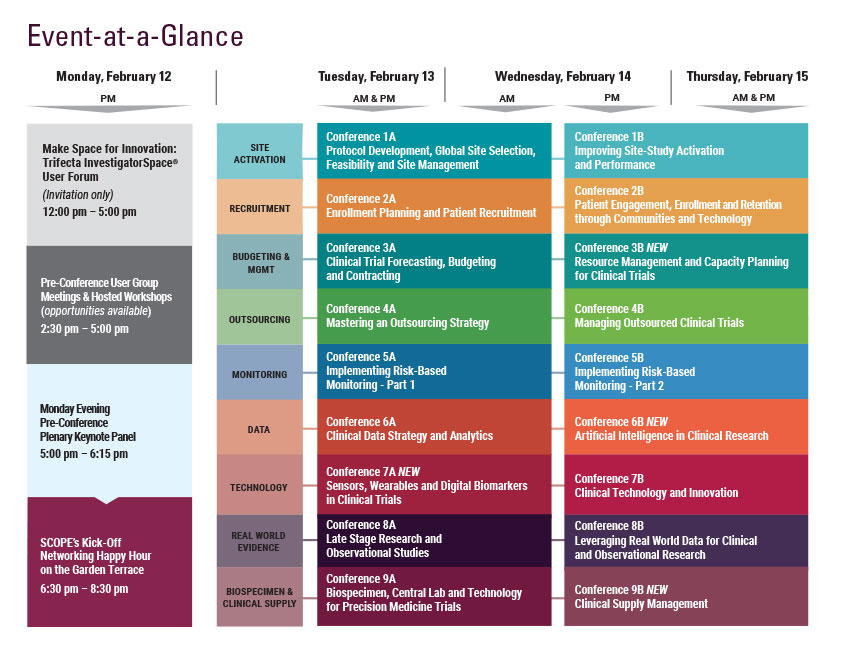
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Cathy Critchlow, Ph.D., Vice President, Center for Observational Research, Amgen
10:50 Evolving Role for Real-World Evidence in Facilitating Regulatory, Payer and Provider Decision-Making
 Cathy Critchlow, Ph.D., Vice President, Center for Observational Research, Amgen
Cathy Critchlow, Ph.D., Vice President, Center for Observational Research, Amgen
Increasing use of real world evidence (RWE) to tailor health care decision-making to patients in clinical practice complements evidence obtained from the carefully selected patients enrolled in randomized clinical trials. While growing data availability
and sophistication of analytic tools have transformed evidence generation, challenges impeding full realization of benefit from RWE remain. Collectively addressing these challenges and advancing suitable use cases will help guide and enable
appropriate impact of RWE.
11:15 Non-Interventional Studies and Pragmatic Clinical Trials to Support Product Value
 Christopher Chinn, Head of RWE Strategy for Market Access Health Economics and Value Assessment,
Sanofi
Christopher Chinn, Head of RWE Strategy for Market Access Health Economics and Value Assessment,
Sanofi
Real World Evidence can be defined to include both non-interventional studies - aka observational studies or registry studies – and pragmatic clinical trials. These can provide evidence of interest to payers and inform clinical guidelines.
The design and delivery of such studies draws on skills required for RCTs, but raise new challenges. Study teams should be prepared to find appropriate solutions.
11:40 Combining Safety, Efficacy and Pharmacoeconomics Endpoints
Durgesh Bhandary, Senior Director, HEOR, AstraZeneca
This talk will discuss the strategy and logistics of designing and executing an observational study that would incorporate multiple end points to serve major stakeholders such as epidemiologists, drug safety researchers, sales and marketing, treating
physicians.
 12:05 pm Next Generation Real-World Evidence: Moving the Industry Standard Forwards
12:05 pm Next Generation Real-World Evidence: Moving the Industry Standard Forwards
Louise Parmenter, PhD, Vice President, Global Head, Scientific Affairs, Real-World Insight Solutions, IQVIA
New Approaches to Generate RWE. Using Data and Insights to Improve Predictability of Delivery.
12:30 Session Break
12:40 Luncheon Presentation: Value of Natural History Studies Throughout the Product Life Cycle
 Haley Kaplowitz, Ph.D., Executive Director, Safety, Epidemiology, Registries and Risk Management, UBC: An Express Scripts
Company
Haley Kaplowitz, Ph.D., Executive Director, Safety, Epidemiology, Registries and Risk Management, UBC: An Express Scripts
Company
Having an in-depth knowledge of the disease or condition for which is drug is being developed seems fundamental, yet the natural history of the disease may not be fully known prior to or during drug development. Further, once a new product enters
the marketplace, it is important to characterize the impact on disease natural history. Successful product development and marketing are aided by the collection of natural history data.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Ginger Spitzer, Executive Director, Foundation of Sarcoidosis Research
2:05 Co-PRESENTATION: Value of Conducting Feasibility Studies in Observational Research
 Mark Price, Senior Director, Surveys and Observational Studies, RTI Health Solutions
Mark Price, Senior Director, Surveys and Observational Studies, RTI Health Solutions
 David Richardson, Project Manager, Surveys and Observational Studies, RTI Health Solutions
David Richardson, Project Manager, Surveys and Observational Studies, RTI Health Solutions
This presentation will provide justification and case study examples to demonstrate why it is important to spend time and resources up front to conduct feasibility assessments in noninterventional studies and the range of feasibility approaches
that could be performed to get the most out of the implementation phase.
2:30 CO-PRESENTATION: Collaborative Approach to Real World Data Collection
 Ginger Spitzer, Executive Director, Foundation of Sarcoidosis Research
Ginger Spitzer, Executive Director, Foundation of Sarcoidosis Research
 Winnie Nelson, Pharm.D., MS, MBA, Senior Director HEOR, Mallinckrodt Pharmaceuticals
Winnie Nelson, Pharm.D., MS, MBA, Senior Director HEOR, Mallinckrodt Pharmaceuticals
This presentation will focus on the valuable role of non-profit disease research foundations in securing and managing real world data and collaboration with industry to access the data. The two-part presentation will include review of
methods such as patient registries, clinical site networks, collaboration, wearables, and other techniques, and will feature the perspective of industry partners as both collaborators and first-line collectors of data. The “neutral
third party” status of the non-profit organizations can enable industry to navigate more easily the issues in logistics to get direct real world data.
2:55 A Crawl, Walk, Run Strategy towards Virtual Studies in Real World Research
Adam Halbridge, Vice President, Business Development, Parallel6, a PRA Health Sciences Company
This presentation will address new and exciting developments in planning and executing real world research studies virtually. Attendees will learn about the reality of conducting virtual studies on a global scale and the role mobile
technology can have to optimize recruitment, engagement and retention of patients, while driving better data, decisions and outcomes more efficiently, and at a significant reduction in costs.
3:20 PANEL DISCUSSION: Meeting the Evidentiary Needs of Multiple Stakeholders by Better Non-Interventional Studies
Moderator:
 Cathy Critchlow, Ph.D., Vice President, Center for Observational Research,
Amgen
Cathy Critchlow, Ph.D., Vice President, Center for Observational Research,
Amgen
Panelists: Ginger Spitzer, Executive Director, Foundation of Sarcoidosis Research
Juliane Mills, Scientific Affairs Director, Real World Solutions, PRA Health Sciences
Christopher Chinn, Head of RWE Strategy for Market Access Health Economics and Value Assessment, Sanofi
Topics to be discussed include but are not limited to the following:
- What are key considerations and approaches to balance scientific and commercial values of non-interventional studies?
- What are common utilizations of non-interventional studies in supporting clinical development program?
- How can evidences generated from non-interventional studies be used in discussions with regulatory agencies during product development and post marketing in support of establishing product benefit risk profile, continual safety
monitoring, and risk management and mitigation activities, as well as fulfilling regulatory post marketing safety requirement (PMRs and FUMs)?
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table
of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group
interrogation and problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution
can remove such concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight
best practice technology and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Haley Kaplowitz, Ph.D., Executive Director, Safety, Epidemiology, Registries and Risk Management, UBC: An Express Scripts Company
8:30 CASE STUDY: Leveraging Real World Data in Observational Research to Address Safety Risks
Younus Muhammad, Director, Epidemiology, Worldwide Safety and Regulatory, Pfizer
Observational data from real-world setting are increasingly being used to assess safety risks with pharmaceutical products. This presentation will describe how real world data from routine clinical practice were used to address
a safety risk in a special patient population including details around feasibility assessment, regulatory agency interactions, study methods and results, and impact on the product label.
9:00 CO-PRESENTATION: The Use of Real-World Data and Evidence in Clinical Research and Post-Marketing Safety Monitoring
 Mary Jo Lamberti, Ph.D., Senior Research Fellow, Tufts CSDD, Tufts University
Mary Jo Lamberti, Ph.D., Senior Research Fellow, Tufts CSDD, Tufts University
 Jill Abell, MPH, Ph.D., Team Leader, Real World Evidence, Janssen
Jill Abell, MPH, Ph.D., Team Leader, Real World Evidence, Janssen
Tufts CSDD has recently conducted a study of 30 biopharmaceutical companies to examine insights on the industry’s response to this opportunity including current and planned uses of real-world data, operational issues
and return on investment/performance areas impacted by real-world data use. Significant challenges are identified as well as strategies and practices that impact return on investment or performance.
9:40 Real World Evidence: Separating the Hype from the Promise
 Charles Makin, Vice President & Global Head, Late Phase Research, Commercialisation
& Outcomes, ICON
Charles Makin, Vice President & Global Head, Late Phase Research, Commercialisation
& Outcomes, ICON
Increasing availability of RWE has created justifiable excitement, accompanied by confusion about its true meaning and implications for stakeholders. While RWE is neither the starting point nor the solution, it is a critical
component of the value demonstration toolkit. It is not the data that counts, but the insights gained from it. Join us for best practices to ensure you are asking the right questions and using data to guide the pursuit
of answers.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Maria Harrison, Vice President, Real World Solutions, PRA Health Sciences
11:15 Incorporating the Patient Perspective into Drug Development/Real-World Evidence
 Emily Freeman, Ph.D., Director,HEOR- Patient Reported Outcomes, Abbvie
Emily Freeman, Ph.D., Director,HEOR- Patient Reported Outcomes, Abbvie
Patient engagement is a key aspect to improving health outcomes and contextualizing real-world evidence within the pharmaceutical industry. It is imperative to incorporating the patient perspective into drug development
beyond patient reported outcomes and subsequent RWE strategies. This session strategizes how to incorporate the patient perspective to develop patient focused drug development activities utilizing concepts from the
social/behavioral sciences that focuses on patient activation, patient measurement, and shared-treatment decision making.
11:40 CO-PRESENTATION: Using Exit Interviews with Participants in Randomized Controlled Trials to Collect “The Rest of the Story”
 Carla Romano, MS, Executive Director, Patient Centered Outcomes Assessment, RTI Health
Solutions
Carla Romano, MS, Executive Director, Patient Centered Outcomes Assessment, RTI Health
Solutions
Sandy Lewis, BSN, Director, Patient Centered Outcomes Assessment, RTI Health Solutions
This presentation will explore the benefits of conducting qualitative interviews with patients as they complete participation in RCTs. Important information can be collected on safety signals, clinically meaningful change
on PRO measures, understanding reasons for drop out, and bringing the patient voice into clinical trials.
12:05 pm Session Break
 12:10
pm Bridging Luncheon Presentation: Achieving Evidentiary Equilibrium
12:10
pm Bridging Luncheon Presentation: Achieving Evidentiary Equilibrium
 David Thompson, Ph.D., Senior Vice President, Real-World & Late Phase, Syneos Health
David Thompson, Ph.D., Senior Vice President, Real-World & Late Phase, Syneos Health
Achieving Evidentiary Equilibrium - Generating the Right Evidence for the Right Stakeholders at the Right Time Throughout the Clinical/Commercial Continuum
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech
Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute
(CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5456
E: rhandy@healthtech.com