Cambridge Healthtech Institute’s 5th Annual
Patient Engagement, Enrollment and Retention through Communities and Technology:
Patient Centric Approaches to Optimize Clinical Trials and Participant Engagement
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
Enrollment planning and patient recruitment are critical to drug development programs and garner a lot of attention by study teams. However, once the hard work of identifying and recruiting a trial subject has been accomplished they must be retained and
remain in compliance. Retention of patients throughout the life of a clinical trial is essential in order to have complete data sets for your analysis and subsequent filings. There are strategies, tools and techniques such as social media platforms
and mobile technology, empowered patient communities, and a more informed patient population that need to be understood and engaged. CHI’s 5th Annual Patient Engagement, Enrollment and Retention through Communities and Technology will cover the topics one should consider when planning and strategically implementing a patient retention plan in the digital age.
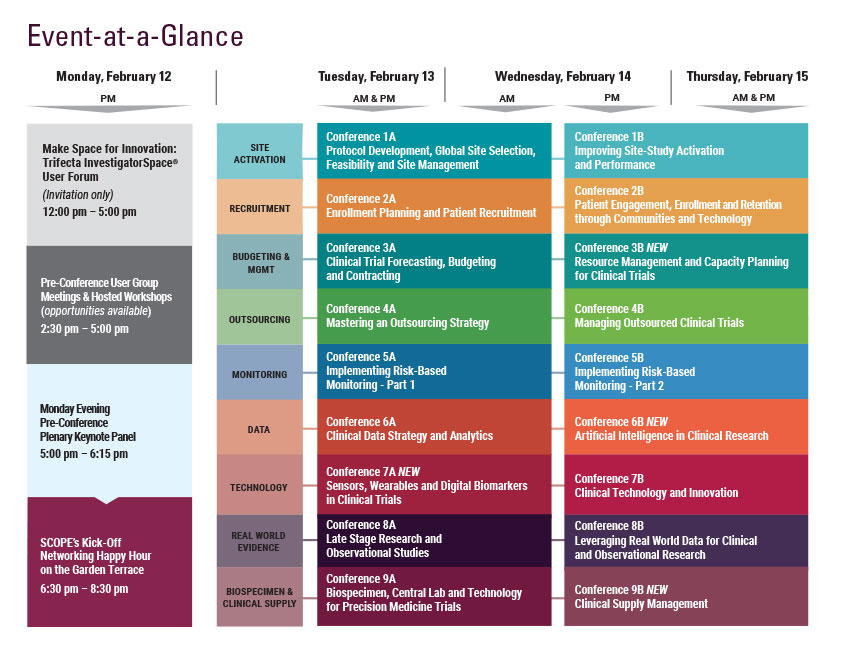
Wednesday, February 14
11:30 am Registration Open
 12:10 pm Bridging
Luncheon: Taking Patient Recruitment into the Next Generation of Clinical Development
12:10 pm Bridging
Luncheon: Taking Patient Recruitment into the Next Generation of Clinical Development
Kimberly Ray, Vice President, Site and Patient Networks, IQVIA
Using real-world insights to drive better Site identifications. Understanding Site and PI capabilities to drive ideal patients. The importance of communication and advocacy groups to drive engagement and retention.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Terrie Livingston, Pharm.D., Senior Director, Real World Outcomes, Innovative Partnerships & Insights (RI2), Biogen
4:05 Top 10 Rules of Engagement to Incorporate the Voice of the Patient in Clinical Development
 Paulo Moreira, Vice President, Global Clinical Operations - External Innovation, EMD Serono
Paulo Moreira, Vice President, Global Clinical Operations - External Innovation, EMD Serono
The industry is making strides in its attempt to include the voice of the patient in clinical development, but much uncertainty on how to do it still exists. Successful engagement between pharma and patients requires that some basic tenets be observed.
This presentation will enumerate and discuss the top 10 rules based on several years of engaging patients to seek their input into clinical trial design and protocol operational implementation.
4:30 Developing the Conversation around Guardrails for Patient Information Gathered in Patient Engagement Activities
 Therese Johnsen, Clinical Trial Intelligence Manager, Global Drug Development, Novartis
Therese Johnsen, Clinical Trial Intelligence Manager, Global Drug Development, Novartis
A little studied topic is how companies and vendors are protecting the patient information gathered during engagement activities. Data Privacy rules protect a certain type of data depending on the country, but contracts can give wide access to patient
data which can be used in a myriad of ways and travel the world in global clinical trial teams. Sponsors spend a lot of effort on governance, compliance and quality, often more focused on protecting the company. What guardrails are needed across the
industry to ensure that people that share their data during partnerships in drug development do not experience unintended outcomes.
4:55 CO-PRESENTATION: Easing the Rare Disease Patient’s Burden in Clinical Trials with Innovative Technology
 Scott Schliebner, MPH, Vice President, Rare Diseases, Scientific Affairs, PRA Health Sciences
Scott Schliebner, MPH, Vice President, Rare Diseases, Scientific Affairs, PRA Health Sciences
 David Turner, MS, CISA, CEO/Founder, Parallel6, a PRA Health Sciences Company
David Turner, MS, CISA, CEO/Founder, Parallel6, a PRA Health Sciences Company
A rare disease patient’s diagnostic journey can be overwhelmingly arduous and expensive, so participating in clinical trials may seem especially burdensome. Traditional clinical trial models for screening, enrollment and ongoing study requirements
may not be desired, possible or practical. Join this presentation to learn how a patient-centric approach, that provides an end-to-end solution and seamlessly integrates into a patient’s mobile lifestyle, may increase patient recruitment, enrollment
and engagement.
5:25 CASE STUDY: Plain Language ICF Project
 Suzann Johnson, Associate Director, Investigator and Patient Engagement Projects, Global Clinical Development Operations,
Janssen R&D
Suzann Johnson, Associate Director, Investigator and Patient Engagement Projects, Global Clinical Development Operations,
Janssen R&D
Informed Consent is one of the most important aspects of the clinical research process; it is also one of the areas that most sponsor companies struggle with, as the document is heavily regulated by the regulations and internal compliance/legal groups.
Janssen R&D is undergoing a project to overhaul our ICF to make it a more effective and patient-centric document.
 5:50 Reception Hosted by Cognizant Technology Solutions
5:50 Reception Hosted by Cognizant Technology Solutions
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation, we will
go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
Daniel Piekarz, Senior Vice President, Healthcare & Life Sciences Practice Leader, DataArt
8:35 CASE STUDY CO-PRESENTATION: What Patients Want You to Know: Treat Them Like Consumers
 Brieana Cox-Buckley, Pharm.D., Executive Director, US Field Medical and Value Based Outcomes, Bioverativ
Brieana Cox-Buckley, Pharm.D., Executive Director, US Field Medical and Value Based Outcomes, Bioverativ
.jpg) Christopher O’Brien, Vice President, Strategic Partnerships, myHealthTeams
Christopher O’Brien, Vice President, Strategic Partnerships, myHealthTeams
More and more patients have become reliant upon and trust online information and they have access to more information than ever before. What have we learned from the patient communities, and how can we apply those insights into better outcomes for our
clinical trials? This co-presentation will share a case study with a specific example from a hemophilia patient population.
9:00 Inclusion of Patient Reported Outcomes (PROs) in Rare Disease Trial Design: How Social Networks Can Enhance Patient Engagement and Patient Experience
 Terrie Livingston, Pharm.D., Senior Director, Real World Outcomes, Innovative Partnerships & Insights
(RI2), Biogen
Terrie Livingston, Pharm.D., Senior Director, Real World Outcomes, Innovative Partnerships & Insights
(RI2), Biogen
This presentation is given from the perspective of someone who understands clinical research both as a member of industry, but also as a patient. It will cover: Developing meaningful patient reported outcomes by gathering information about their disease
journey through an independent social network, whether PROs will be used to run 3b trials to produce further evidence to support approval, strategies and tools to enhance patient engagement and patient experience.
9:25 PANEL DISCUSSION: Understanding the Power of Social Networks to Facilitate Engagement, Education, Research and Recruitment
Moderator:
 Pablo Graiver, Co-Founder & CEO, Antidote
Pablo Graiver, Co-Founder & CEO, Antidote
 Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical Operations,
Bristol-Myers Squibb
Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical Operations,
Bristol-Myers Squibb
 Eric Peacock, Co-Founder & CEO, myHealthTeams
Eric Peacock, Co-Founder & CEO, myHealthTeams
 Gilles Frydman, Patient Advocate; Co-Founder Smart Patients and ACOR (Asssociation of Cancer Online Resources)
Gilles Frydman, Patient Advocate; Co-Founder Smart Patients and ACOR (Asssociation of Cancer Online Resources)
 Terrie Livingston, Pharm.D., Senior Director, Real World Outcomes, Innovative Partnerships &
Insights (RI2), Biogen
Terrie Livingston, Pharm.D., Senior Director, Real World Outcomes, Innovative Partnerships &
Insights (RI2), Biogen
- How can patient communities empower patients and enable research?
- What do trial designers, patient recruitment experts, protocol writers, and patient centricity groups need to better understand about both the technology and the relationships?
- What are the key challenges and opportunities of using social media tech and social networks to better engage patients?
9:50 Finding the "Center" Of Your Clinical Trial
 Jonathan Andrus, MS, CQA, CCDM, COO, Clinical Ink
Jonathan Andrus, MS, CQA, CCDM, COO, Clinical Ink
This presentation will focus on ways that you can center your clinical trial on the needs of the patient and the site throughout your clinical trial. Considerations during the start up, conduct and closeout phase will be examined. Further, attendees will
learn about ways to engage with clinical trial patients in ways that enable better retention and engaged involvement in the study.
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Heather Hernandez, Director, Business Development, Seeker Health
10:35 CO-PRESENTATION: Inside Out: A New Approach to Patient Engagement
 Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical
Operations, Bristol-Myers Squibb
Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical
Operations, Bristol-Myers Squibb
 Mary Murray, Associate Director, Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
Mary Murray, Associate Director, Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
Patient engagement does not always look and feel the same from internal and external perspectives. How can patient engagement strategies be developed to perpetuate an ongoing and robust conversation with patients as partners? The speakers will address
this question, sharing examples and unexpected experiences from the past few years that have led to the development of a new approach.
 11:00 Patient Engagement Strategies to Increase Clinical Study Awareness, Interest, and Referrals Across Indications
11:00 Patient Engagement Strategies to Increase Clinical Study Awareness, Interest, and Referrals Across Indications
 Barbara Zupancic, Senior Director, Global Patient Recruitment and Retention, Worldwide Clinical Trials
Barbara Zupancic, Senior Director, Global Patient Recruitment and Retention, Worldwide Clinical Trials
Within patient communities certain populations have become more willing to embrace clinical trial communications through digital channels including social media. However, others remain hesitant when it comes to receiving information about sensitive
health issues on platforms such as Facebook. This session will examine the benefits and best practices of social media while addressing other “off-line” strategies that have shown practical results in reaching prospective participants
who prefer traditional communication channels.
11:25 Transition to Shared Session
11:35 Digital Trends Impacting Recruitment, Engagement and Retention
 Shwen Gwee, Head of Digital Strategy, Global Clinical Operations, Biogen
Shwen Gwee, Head of Digital Strategy, Global Clinical Operations, Biogen
Digital technology is connecting more people to clinical trials than ever before, and at the same time the adoption of wearables as data collection devices in clinical trials is rising. The hope of streamlining trial operations, patient recruitment
and registration is real. What technologies and approaches are having the greatest impact on recruitment, engagement and retention?
12:00 pm CASE STUDY: The Art and Science of Patient Engagement in the Digital Era – Learning from GSK PARADE Study
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
The ability to efficiently develop new medicines for patients with unmet needs is limited by the current model for clinical development. Although the emerging mHealth technologies have the potential to improve the conduct of clinical trials, the successful
implementation requires careful study design and patient engagement. Data and experiences from GSK PARADE study will be summarized, highlighting the learning and opportunities in this new area of clinical development.
12:25 Mobile Clinical Trials: New Findings on Patient and Site Perspectives from the Clinical Trials Transformation Initiative
 Hassan Kadhim, Business Consultant for Clinical Operations, Boehringer Ingelheim Pharmaceuticals
Hassan Kadhim, Business Consultant for Clinical Operations, Boehringer Ingelheim Pharmaceuticals
This presentation will discuss qualitative and quantitative research conducted by the Clinical Trials Transformation Initiative to understand the perspectives of patients and site investigators of mobile clinical trials. Discussion will include
insights and advice from site investigators who have participated in trials that incorporate mobile devices to collect data for study endpoints.
12:50 PANEL DISCUSSION: Why Are There Barriers to the Adoption of Innovative Processes and Technologies at Sites?
Moderator:
 Jim Kremidas, Executive Director, Association of Clinical Research Professionals (ACRP)
Jim Kremidas, Executive Director, Association of Clinical Research Professionals (ACRP)
 David Vulcano, Assistant Vice President & Responsible Executive for Clinical Research, Hospital Corporation
of America (HCA)
David Vulcano, Assistant Vice President & Responsible Executive for Clinical Research, Hospital Corporation
of America (HCA)
 Sean Walsh, MBA, Chief Development Officer, Raleigh Neurology Associates
Sean Walsh, MBA, Chief Development Officer, Raleigh Neurology Associates
 Beth Harper, MBA, Workforce Innovation Officer, Association of Clinical Research Professionals (ACRP)
Beth Harper, MBA, Workforce Innovation Officer, Association of Clinical Research Professionals (ACRP)
Many innovative technologies and process improvement initiatives are coming out at a rapid pace, whether from TransCelerate and other industry consortia, or from technology companies themselves. Which of these improvements actually work? How can
sites implement these more effectively? Why are there barriers to adoption, and how can the innovators better understand sites’ needs?
- Share sites’ perspective on the evolving clinical research landscape
- Discuss the reasons sites struggle with new processes and technology tools
- Determine ways to facilitate adoption
1:15 Closing Remarks
1:20 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for multiple attendees from
the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees from
the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Micah Lieberman
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E:
rhandy@healthtech.com