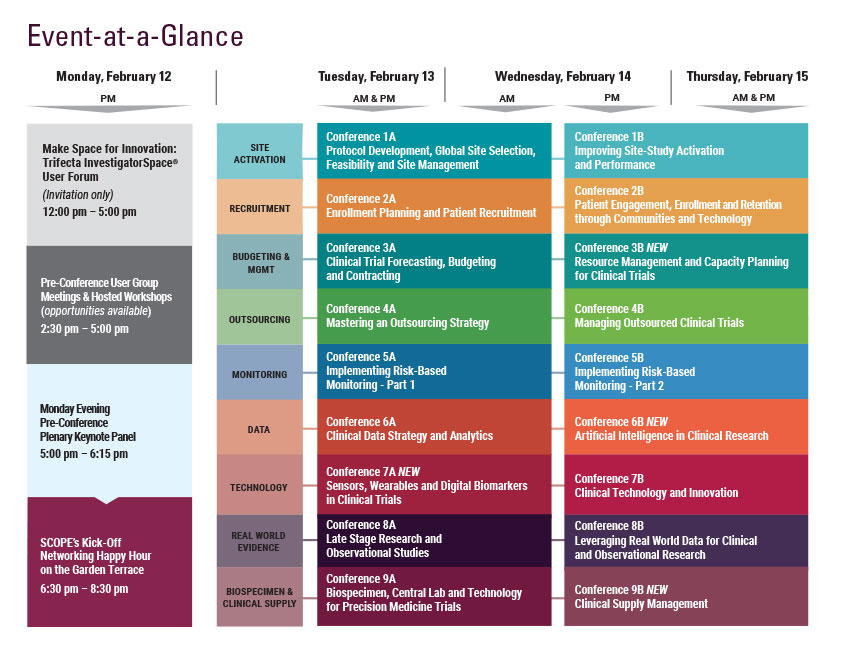
Cambridge Healthtech Institute’s 4th Annual
Implementing Risk-Based Monitoring – Part 2:
Ensuring Effective and Efficient Monitoring and Data Quality
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
Risk-based monitoring (RBM) approaches promise to improve clinical trial efficiency while ensuring data quality. As industry adoption of RBM increases, it is clear that although RBM takes many forms – remote, centralized, and risk-based monitoring
– successful risk-based monitoring implementation requires developing new roles, analytics and processes among the stakeholders in RBM. Cambridge Healthtech Institute’s 4th Annual Implementing Risk-Based Monitoring – Part 2: Ensuring Effective and Efficient Monitoring and Data Quality conference
offers case studies and practical solutions from across pharma and TransCelerate member organizations on effectively implementing clinical quality and RBM as well as a prospective look into the future of RBM.
Wednesday, February 14
11:30 am Registration Open
12:10 pm Bridging Luncheon Presentation: Extracting Data from the EHR Dramatically Reduces the Need for Manual Monitoring - New Standards Make this Possible
 Kim Rejndrup, Senior Vice President, Product Development, OmniComm
Kim Rejndrup, Senior Vice President, Product Development, OmniComm
The typical workflow at an investigate site is for the patient data to be entered into the Electronic Health Record, then for the study coordinator to re-type that data into the EDC system. A big component of the subsequent Source Data Verification is
simply checking that the retyping was done accurately. If data could instead flow automatically from the EHR system, with the coordinator just verifying the data in transit, then both the data entry and the subsequent SDV can be automated. New standards
and technologies being introduced under the “SMART on FHIR” umbrella now make this possible. This presentation will describe the state of the art in this exciting new field.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
David Lacagnina, Technology Entrepreneur, The e-Clinical Agency
4:05 PANEL DISCUSSION: A Cross-Functional Look at RBM from Abbott Nutrition’s RBM Task Force
Moderator:
 Sonya Verrill, Manager, Clinical Projects, Clinical Operations, Abbott Nutrition
Sonya Verrill, Manager, Clinical Projects, Clinical Operations, Abbott Nutrition
Panelists:
 Geraldine Baggs, Ph.D., Principal Research Statistician, Statistical Sciences, ANRD Scientific and Medical Affairs,
Abbott Nutrition
Geraldine Baggs, Ph.D., Principal Research Statistician, Statistical Sciences, ANRD Scientific and Medical Affairs,
Abbott Nutrition
 Dione Smart, Research Data Coordinator, Clinical Data Management, Abbott Nutrition
Dione Smart, Research Data Coordinator, Clinical Data Management, Abbott Nutrition
 Xiaosong (Sue) Zhang, MS, MAS, Staff Statistical Analyst, Clinical Program & System Support, Abbott Nutrition
Xiaosong (Sue) Zhang, MS, MAS, Staff Statistical Analyst, Clinical Program & System Support, Abbott Nutrition
Abbott Nutrition has established a cross-functional RBM task force to address changes brought about by implementing RBM. Members from the following groups: Stats, ClinOps, Programming, and CDM will discuss change management, their successes and challenges,
refinements and tools in their journey to implementing RBM.
 4:55 Think
You’re ICH E6 Compliant? Show Me the Quality Tolerance Limits
4:55 Think
You’re ICH E6 Compliant? Show Me the Quality Tolerance Limits
 Dan O'Connell, Principal, RBM Platform Adoption, Medidata
Dan O'Connell, Principal, RBM Platform Adoption, Medidata
ICH E6 Good-Clinical-Practice Guideline addendum directs sponsors to decide which risks to reduce and/or which to accept, predefining quality tolerance limits(QTLs), taking into consideration the medical/statistical characteristics of the variables and
the statistical design of the trial, to identify systematic issues that can impact subject safety or reliability of trial. The presentation will cover history behind QTLs, difference between QTLs and KRIs and mechanisms to establish, track and report
deviations of QTLs in the CSR.
5:25 RBM Journey: RBM 1.0 to RBM 2.0 with Case Study on a Pilot
 Nurcan Coskun, Ph.D., Global Risk Based Monitoring Program and Technology Solutions Manager, Medtronic
Nurcan Coskun, Ph.D., Global Risk Based Monitoring Program and Technology Solutions Manager, Medtronic
This presentation will discuss: 1. Harmonization of two organizations RBM methodology in Medical Device setting, 2. Future state process development with tools and transition to system and technology solutions, 3. Sharing a case study of RBM 2.0 pilot
implementation, and 4. The challenges around change management and getting everyone aligned to the same direction in a multi-BU company like ours as part of the journey.
 5:50 Reception Hosted by Cognizant Technology Solutions
5:50 Reception Hosted by Cognizant Technology Solutions
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation, we
will go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
8:35 Best Practices and Observations from Implementing TransCelerate’s Risk-Based Monitoring Model Framework
 Suzanne Lukac, Director, Risk-Based Monitoring Implementation, Merck
Suzanne Lukac, Director, Risk-Based Monitoring Implementation, Merck
Although Regulators were urging companies to move to a risk-based approach, no model framework existed to enable organizations to successfully deploy and scale risk-based monitoring. To address this, a collaboration of 18+ Sponsor Companies worked
in an unprecedented way to develop a model approach for risk-based monitoring. This session will share the latest work of this initiative, including new tools to assist implementation, best practices for adoption, and post-adoption metrics and
observations.
9:00 Developing a Risk Repository
 Adrienne Strickler, Associate Director, Risk Management-Central Monitoring, Janssen
Adrienne Strickler, Associate Director, Risk Management-Central Monitoring, Janssen
What comes next after your Risk-Based Monitoring process is established? Process improvement! After 4 years of RBM at Janssen R&D, our portfolio has grown to include well over 150 trials and we are now able to gain efficiencies by applying lessons
learned across similar studies. One key way to do so is through a central risk repository that can be used to streamline RBM set-up and implementation for new trials.
9:25 CO-PRESENTATION: The Quality Journey - A Small Companies Approach to the Implementation of ICH E6 and RBM
 Yiwen Sun, Senior Clinical Research Associate, Samumed, LLC
Yiwen Sun, Senior Clinical Research Associate, Samumed, LLC
 Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
This presentation will be in two parts, firstly examining the overall quality imperative within the clinical trial arena and the second part will focus on Samumed’s ICH E6 and RBM journey to current status and future direction. Key points: 1.
Understand the quality requirement expected of sponsors, 2. What can be achieved by a small company, current status, and 3. Future direction.
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
10:35 RBM: A Larger Sponsor’s Approach
 Taras Carpiac, Director, Global Development Operations, Amgen
Taras Carpiac, Director, Global Development Operations, Amgen
Amgen began its RBM journey in 2012. In the years since, Amgen has continued to make investments in its RBM process and tools in order to align with regulations and in response to industry developments. This presentation will examine the principles
of Amgen’s RBM model and highlight areas of new focus.
 11:00 CO-PRESENTATION: The Right Ingredients for RBM Success
11:00 CO-PRESENTATION: The Right Ingredients for RBM Success
 Danilo Branco, RBM Lead, Senior Manager, Monitoring & Data Flow Optimization, Covance
Danilo Branco, RBM Lead, Senior Manager, Monitoring & Data Flow Optimization, Covance
 Aaron Mackey, PhD, Director, Data Sciences, Covance
Aaron Mackey, PhD, Director, Data Sciences, Covance
Choosing a RBM implementation plan is like following a cooking recipe – step by step or via experience! Hear from two of our experts as they discuss the RBM landscape for today and tomorrow, and offer real word experiences of RBM. Together,
they will provide you with a recipe for success from both a theoretical and operational aspect.
11:25 Brief Session Break
 11:35 The Past, Present and Future of RBM Technology
11:35 The Past, Present and Future of RBM Technology
 Kristin Mauri, Global Head, Risk Based Monitoring, Bioclinica
Kristin Mauri, Global Head, Risk Based Monitoring, Bioclinica
12:00 pm PANEL DISCUSSION: What Lies in the Future for RBM?
Moderator:
 Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
Panelists:
 Yiwen Sun, Senior Clinical Research Associate, Samumed, LLC
Yiwen Sun, Senior Clinical Research Associate, Samumed, LLC
 Taras Carpiac, Director, Global Development Operations, Amgen
Taras Carpiac, Director, Global Development Operations, Amgen
 Andrew Chapman, Senior Director, Informatics & Digital Marketing, Covance
Andrew Chapman, Senior Director, Informatics & Digital Marketing, Covance
 Kristin Mauri, Global Head, Risk Based Monitoring, Bioclinica
Kristin Mauri, Global Head, Risk Based Monitoring, Bioclinica
As the biopharma industry perfects the process of RBM, we will discuss the possibilities that lie ahead for RBM and its resulting data: In what capacity will onsite monitoring continue? Can onsite monitoring ever be eliminated? What are some possible
uses for the data generated from RBM? Are there data patterns and predictive markers seen in the data?
12:50 Closing Remarks
1:00 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for multiple attendees from the
same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees from the
same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com