Cambridge Healthtech Institute’s 8th Annual
Clinical Trial Forecasting, Budgeting and Contracting:
Innovative Strategies for Cost-Efficient Trials
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
There are many reasons a clinical trial can be delayed, but many of these can be prevented through proper forecasting, budgeting, and contracting strategies. As clinical trials become more complex, with a number of CROs, third-party vendors, and sites
getting involved, sponsors must evolve their strategies and take advantage of technology to streamline these processes and set clear expectations from the get-go. Cambridge Healthtech Institute’s 8th Annual Clinical Trial Forecasting, Budgeting and Contracting conference shares case studies and best practices on effective budgets and clear contracts, as well as metrics and key performance indicators of their success.
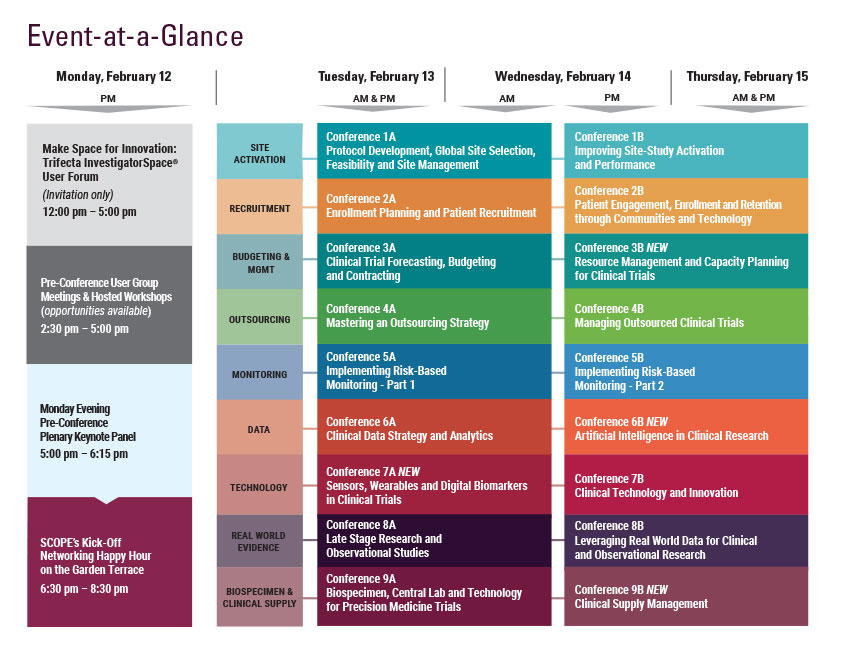
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Chris Chan, Executive Director, R&D Finance, Finance, FibroGen, Inc.
10:50 Budget Forecasting and Tracking: Teamwork and Transparency
 Kenneth Olovich, Chief Financial and Procurement Officer, Chorus
Kenneth Olovich, Chief Financial and Procurement Officer, Chorus
This talk will discuss how the application of budgetary and invoicing models can lead to increased trust. What do sponsors really need to help them manage CRO and trial related expenses? Some budgetary models require more effort to set up than
others; when is it worth the investment? The timing of spend and the accurate projection of the same is just as important as the total spend -- this talk will discuss finding the balance.
11:15 Cost, Time, and Quality Trade-Off in Clinical Trials
 Ozgur Ozkan, Decision Science Director, Biometrics and Information Sciences, AstraZeneca Pharmaceuticals
Ozgur Ozkan, Decision Science Director, Biometrics and Information Sciences, AstraZeneca Pharmaceuticals
This presentation will report on a novel approach to estimate trial costs at the country level and how it is used within a simulation tool to visualize cost, time and quality trade-offs between alternative recruitment scenarios. We will give an
overview of the analysis on the operational/financial data and demo the tool to show how it informs decision making in Clinical Operations.
11:40 CO-PRESENTATION: Clinical Trial Budgeting/Forecasting SMACK-DOWN: Investigator Sites vs. Sponsors – Budgeting Issues
 Chris Chan, Executive Director, R&D Finance, Finance, FibroGen, Inc.
Chris Chan, Executive Director, R&D Finance, Finance, FibroGen, Inc.
 Al Peters, President, Clinical Operations and Finance, BTC Network
Al Peters, President, Clinical Operations and Finance, BTC Network
Although theoretically teammates with common goals, when Sponsors and Investigator Sites interact over budget and money issues, conflicts often arise in the form of miscommunications or even animosity that would make the Hatfields and McCoys blush.
This session will explore some common conflicts as well as possible resolutions..
12:05 pm The RX for Your Clinical Financial Management
 Nina Pruitt, Senior Product Marketing Director, Medidata
Nina Pruitt, Senior Product Marketing Director, Medidata
Upfront study design, grant budgeting, and an investigator payment strategy are foundational for comprehensive clinical financial management. Technology and historical data enable effective scenario planning to ensure your study: achieves desired
endpoints and optimal outcomes, results in appropriate, defendable site budgets and delivers tight financial management for all stakeholders. Excel simply cannot deliver for the financial management requirements for todays’ clinical
research. Top pharma and CROs have moved away from excel – have you?
12:30 Session Break
 12:40 Luncheon Presentation: An Easy 4-Step Process That Will Make You a CTMS Evaluation Hero
12:40 Luncheon Presentation: An Easy 4-Step Process That Will Make You a CTMS Evaluation Hero
 Jens B. Thuesen, CTMS Business Development, BSI Business Systems Integration AG
Jens B. Thuesen, CTMS Business Development, BSI Business Systems Integration AG
Is your CTMS evaluation process outdated? Using scorecards to evaluate solutions based on features alone can be frustrating. And knowing the right questions to ask that go beyond basic features can be daunting. You don’t have to be an IT
expert to choose a CTMS that will serve you well for years to come. Learn how a concise 4-step process can get you on your way to a solution that fits your organization perfectly.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations and Compliance, Boston University
2:05 Building a Per Patient Cost and, Ultimately, the Entire Investigator Grant Budget
 Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
In this session, we will look at a practical approach for establish a preliminary site budget template, and how this rolls into establishing the entire investigator grant budget. Standard of Care plays an important role in the US, and some countries;
this needs to be factored into the budget templates. When all is complete for the site level template, we will evaluate how this can be utilized to build a forecast for total investigator grants and what is factored into these calculations.
We will also look at how this feeds into a typical full clinical trial budget and forecast.
2:30 CO-PRESENTATION: Merck’s Site Ready Team: Integrating Capabilities to Provide a Centralized Approach and User Experience for Investigators Budget and Payment Process
 Cathy Carfagno, Associate Director, MRL IT, Merck & Co., Inc.
Cathy Carfagno, Associate Director, MRL IT, Merck & Co., Inc.
 Rochelle Redding, Associate Program Manager, MRL, Merck & Co., Inc.
Rochelle Redding, Associate Program Manager, MRL, Merck & Co., Inc.
This talk will present an overview of the strategy that Merck would like to take in changing its global budget and payment process and our thoughts on enabling a more integrated collaboration and interaction with our investigative sites. In order
to maintain strong and effective investigator relations and to enhance our site performance, while ensuring meeting FDA compliance rules, we have started to map out a simplified process for creating and negotiating our budgets as well as improving
our payment controls, all while operating in a very budget-conscious environment.
2:55 PANEL DISCUSSION: Budgeting with Sites: Bottlenecks, Challenges, and Opportunities
Moderator:
 Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Panelists:
 Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations
and Compliance, Boston University
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations
and Compliance, Boston University
 Kenneth Olovich, Chief Financial and Procurement Officer, Chorus
Kenneth Olovich, Chief Financial and Procurement Officer, Chorus
 Rochelle Redding, Associate Program Manager, MRL, Merck & Co., Inc.
Rochelle Redding, Associate Program Manager, MRL, Merck & Co., Inc.
During site budget negotiations, don’t you often want to just ask the other party “Are you crazy or just being stubborn?” We all have different end games during negotiations, and it’s important to understand the dynamics
of the Sponsor, Site and CRO triad. As this triad continues to be dominant in our industry, it’s important that we learn to predict each other’s reactions in order to avoid “show-down” meetings where all are frustrated
and ready to walk away. We all think we are right, but ultimately, it’s about the final negotiated budget, how we get there, and creating a win-win-win scenario for all.
 3:20 Clinical Payments Case Studies: Improving Efficiency, Cash Management, and Compliance
3:20 Clinical Payments Case Studies: Improving Efficiency, Cash Management, and Compliance
 Meghan Harrington, Vice President, Operations, Financial Lifecycle Solutions, Bioclinica
Meghan Harrington, Vice President, Operations, Financial Lifecycle Solutions, Bioclinica
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest
and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem
solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove such
concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice technology and
process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Anca Copaescu, CEO, Strategikon Pharma
8:30 Contracts and Budgeting for Studies Involving Special Populations and Adaptive Designs: Evolving Challenges with Precision Medicine Trials
 Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations
and Compliance, Boston University
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations
and Compliance, Boston University
In-human (FIH) studies now routinely contain several parts with multiple cohorts with increased dose flexibility and accelerated dose escalation paradigms, each of which might have been previously a separate clinical trial. Sponsors, CROs
and investigators struggle to minimize risks and avoid serious consequences, and yet, there remains real risk in an FIH study. Understating challenges associated with the use of special populations is critical to ensure that the study
design will allow for the timely and successful completion of the project, while minimizing individual exposure to the risks of participating subjects, planning and implementing budgets, forecasting, managing costs, and assessing safety
profile.
8:55 Streamlining CTA Negotiations beyond the Legal Language
 Débora Araujo, Associate Director, Site Budgets and Payments (US Group Head), Boehringer Ingelheim
Pharmaceuticals, Inc.
Débora Araujo, Associate Director, Site Budgets and Payments (US Group Head), Boehringer Ingelheim
Pharmaceuticals, Inc.
It is widely known that legal language is one of the main pain points threatening the timely execution of CTAs. The issue has prompted much-needed industry focus and related initiatives in recent years in an effort to standardize legal
language and thus streamline CTA negotiations. However, to effectively address the overall delays in CTA execution, some other aspects of the negotiation process must be dealt with as well. In this presentation, we will explore the
top aspects of the negotiation process threatening an expeditious CTA execution and some practical ways to counter each of them.
9:20 Strategies to Reduce Time to Contract Approval: A Site Perspective
 JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
How can sites accelerate the CTA review process for quicker approval of a fair and balanced contract? This session covers strategies and practical tips to speed up the CTA review and approval process. Topics include preparation, contract
pitfalls, language, contract editing, common contract challenges between the site and sponsor, and leveraging site data and metrics.
 9:45 Driver Based Forecasting - Improving Accuracy and Timeliness of Clinical Trial Financial Forecasts
9:45 Driver Based Forecasting - Improving Accuracy and Timeliness of Clinical Trial Financial Forecasts
 Ramprasad Keshavamurthy, MBA, Senior Manager, Life Sciences Consulting, Cognizant
Ramprasad Keshavamurthy, MBA, Senior Manager, Life Sciences Consulting, Cognizant
The current tools available to Clinical Finance groups make it effort-intensive and reliant on past experience of associates to develop forecasts. Intricacy of study design, global spread of trials, and reliance on third parties add to
the complexity. Learn how driver-based forecasting provides a consistent approach to clinical budgeting and how digital technologies can report on updated protocols, vendor contracts, and trial progress to provide the actual financial
position of R&D budgets in near-real time.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Anca Copaescu, CEO, Strategikon Pharma
11:15 PANEL DISCUSSION: Resource Allocation and Its Effect on Contracting between CROs and Sponsors
 Greg Skalicky, Executive Vice President and General Manager, Syneos Health
Greg Skalicky, Executive Vice President and General Manager, Syneos Health
 Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka
Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka

Tara Dubois, MBA, Head, Clinical Trial Cost Management, Business Operations, Pfizer
Daniel Smith, Vice President, Global Solutions, Kelly OCG
Procurement, contracting, and clinical operations teams need transparency with their CRO partners in order to properly understand a CRO’s allocation of resources and costs, especially when contracting key deliverables. This panel
will address, from the Sponsor’s and CRO’s perspective, resource allocation considerations during the contracting process: potential challenges, what CROs wish pharma knew, and pitfalls to avoid.
12:05 pm Session Break
 12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
 Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
By leveraging site level tooling to surface nearly real-time operational metrics; sponsors and CROs are better equipped to plan for costs associated with individual sites, manage ongoing trial costs and project future budgets.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:

Kaitlin Kelleher
Conference Producer
Cambridge Healthtech Institute (CHI)
T: (+1) 781-972-5498
E: kkelleher@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com